GUIDELINES | https://doi.org/10.5005/jp-journals-10071-23298 |
Practice Guidelines for Enteral Nutrition Management in Dysglycemic Critically Ill Patients: A Relook for Indian Scenario
1Institute of Critical Care and Anesthesiology, Medanta: The Medicity, Gurugram, Haryana, India
2Department of Endocrinology and Diabetology, Institute of Endocrinology and Diabetology, Medanta: The Medicity, Gurugram, Haryana, India
3Department of Anesthesiology, Critical Care and Pain, Tata Memorial Centre, Homi Bhabha National Institute, Mumbai, Maharashtra, India
4Department of Gastrointestinal Surgery, The Institute of Medical Sciences, Care Hospitals, Hyderabad, Telangana, India
5Department of Critical Care Medicine, Artemis Hospital, Gurugram, Haryana, India
6Department of Critical Care Medicine, Sanjeevan and MJM Hospital, Pune, Maharashtra, India
7Department of Intensive Care and Neurotrauma-Stroke Unit, Ruby Hall Clinic, Pune, Maharashtra, India
8Department of Critical Care Medicine, Royal Care Super Specialty Hospital, Coimbatore, Tamil Nadu, India
9Department of Dietetics, Apollo Hospitals, Hyderabad, Telangana, India
10Department of Nutrition and Dietetics, Medica Superspecialty Hospital, Kolkata, West Bengal, India
11,12Department of Scientific and Medical Affairs, Abbott Nutrition International, India
Corresponding Author: Yatin Mehta, Institute of Critical Care and Anesthesiology, Medanta: The Medicity, Gurugram, Haryana, India, Phone: +91 9971698149, e-mail: yatinmehta@hotmail.com
How to cite this article Mehta Y, Mithal A, Kulkarni A, Reddy BR, Sharma J, Dixit S, et al. Practice Guidelines for Enteral Nutrition Management in Dysglycemic Critically Ill Patients: A Relook for Indian Scenario. Indian J Crit Care Med 2019;23(12):594–603.
Source of support: The outcome of a series of two advisory board meetings, three rounds of Delphi voting, and one online survey supported by Abbott Nutrition International, India
Conflict of interest: None
ABSTRACT
Background and aim: Intensive-care practices and settings differ for India in comparison to other countries. While guidelines are available to direct the use of enteral nutrition (EN), there are no recommendations specific to nutritional management of EN in dysglycemic patients, specific to patients in Indian critical care settings. Advisory board meetings were arranged to develop the practice guidelines specific to the Indian context, for the use of EN in dysglycemic critically ill patients and to overcome challenges in this field.
Materials and methods: Two advisory board meetings were organized to review various existing guidelines, meta-analyses, randomized controlled trials (RCTs), controlled trials and review articles, for their contextual relevance and strength. Three rounds of Delphi voting were done to arrive at consensus on certain recommendations. A systematic grading of practice guidelines by the advisory board was done based on strength of the consensus voting and reviewed supporting evidences.
Results: Based on the literature review, the recommendations for developing the practice guidelines were made as per the grading criteria agreed upon by the advisory board. The recommendations were to address challenges regarding prediction and assessment of dysglycemia (DG), acceptable glycemic targets in such settings, general nutritional aspects pertaining to DG nutrition, and nutrition in various superspecialty cases in critical care settings, where DG is commonly encountered.
Conclusion: This paper summarizes the optimum EN practices for managing DG in critically ill patients. The practical solutions to overcome the challenges in this field are presented as practice guidelines at the end of each section. These guidelines are expected to provide guidance for EN management in dysglycemic critically ill patients. These guidelines also outline the model glycemic control task force and its roles in nutrition care as well as an intensive care unit DG nutrition protocol.
Keywords: Blood glucose, Dysglycemia, Glycemic variability, Nutrition.
INTRODUCTION
Critical illness results in various pathophysiological changes like increased inflammation, substrate metabolism, secretion of stress hormones, insulin, growth hormones, and likewise. Certain pharmaceutical interventions such as steroid therapy cause changes in substrate metabolism. Steroids and various drugs have potential to result in the dysglycemic state. Dysglycemia (DG) can worsen the critical illness state, which results in decreased immune function and increased oxidative stress, leading further to a vicious cycle of worsening illness and dysglycemic states.
Patients with DG are predisposed to hospitalization, given the prevalence of comorbid conditions in this population. In-hospital DG is a common finding and needs to be considered an important marker of poor clinical outcomes. Hospitalized patients with DG pose a challenge for the clinician.
Dysglycemia, in the form of hyperglycemia, hypoglycemia, and/or marked glycemic variability (GV), is a characteristic feature of various critical illnesses, irrespective of patient’s diabetic status. Numerous studies have found hyperglycemia, hypoglycemia, and GV as independent risk factors, associated with significant morbidity and mortality.
Severe illness could aggravate or accelerate hyperglycemia-induced cellular damage. Cellular hypoxia upregulates the expression of insulin-independent glucose transporters on the membranes of several cell types. This results in reperfusion injury due to high circulating glucose levels overloading and damaging the cells. In the context of threatened organ function due to critical illness, the DG-induced cellular injury reflects a preventable risk.
Hence, a systematic approach to in-hospital management of DG will certainly demonstrate better patient outcomes and reduced burden on the healthcare system. Apart from pharmaceutical intervention, nutrition care will remain an important aspect for glycemic management in critical care settings.
Despite elaborate literature and protocols that exist for pharmaceutical management of DG, none of the them explicitly discuss the specifics of nutrition intervention in this regard. Indian Practice Guidelines on Critical Care Nutrition by Mehta et al.,1 published in April 2018, was an effort to elaborately discuss critical care EN for Indian settings. However, nutrition in DG in critical care settings was excluded from the ambit of this guideline, since complexities of DG nutrition in Indian ICUs required further discussion. As a continuation to first Indian guidelines on critical care nutrition, the current guidelines address the knowledge gaps in DG nutrition. It is an attempt to bring uniformity in nutritional intervention across diverse critical care settings in India, covering both the resource-intense and the limited-resource settings, while enhancing the standards of overall care. These guidelines may be helpful in other countries with similar healthcare resources.
MATERIALS AND METHODS
An advisory board was constituted with six intensive care specialist physicians, one endocrinologist, one gastrointestinal surgeon, and two clinical nutritionists from nine prominent tertiary healthcare centers across India. The objective was to have a series of advisory board meetings for discussing, deliberating, and reviewing available clinical papers including guidelines, meta-analyses, RCTs, controlled trials, and review articles, published from 1981 to end of 2018 and available on PubMed, Google Scholar, Research gate, and other online medical literature resources, in context of nutritional management of DG in Indian ICUs. The objective was to reach a consensus on India-specific practice guidelines. A pre-meeting online survey, followed by two meetings and three rounds of Delphi voting, was conducted from May 2019 to August 2019.
Grading Criteria
Evidence was graded from A to C, with A as strongest and C as weakest (Tables 1 and 2). On the basis of voting, the recommendations were categorized as I, if agreement was 90% or more, and II, if agreement was between 80% and 90%.
The practice guidelines were finally graded as AI, BI, and CI, which denoted strong recommendation. BI and CI were strong recommendations, despite weak evidence.
The practice guidelines graded as AII, BII, and CII denoted weak recommendation.
Identifying At-risk Patients for Dysglycemic/Hyperglycemia
Undiagnosed DG in hospitalized patients has been well documented as a common in-patient problem and is associated with poor outcomes. Since the prevalence of undiagnosed diabetes mellitus is high in Indian population, a targeted approach for screening unrecognized DG is important.2 Since hospitalization provides a point of contact for high-risk individuals, it may be an opportune time for screening unrecognized DG in such patients. Malcolm et al.3 concluded DG as a common finding in patients admitted for coronary heart disease or elective joint replacement surgery and with no history of diabetes. Various parameters like hemoglobin A1c (HbA1c), random blood sugar, lipid markers, anthropometric measures, and others are being used for diagnosing DG in critically ill patients at the time of admission. Hyperglycemia in a hospitalized patient has been defined as blood glucose (BG) >140 mg/dL. Few studies have evaluated the sensitivity/specificity of using such parameters as the diagnostic markers for DG in critical care settings.4 However, clarity in this aspect is still lacking.
Practice Guidelines
- To improve measuring accuracy, glucometer calibration needs to be frequently done5 AI, as per manufacturer’s recommendations.
- HaA1c equal to or above 8.5% may be taken as an indicator to postpone elective surgeries6 AI, or till the glycemic status is optimized for at least 48 hours. CI
- HbA1c should be done, if random blood sugar (RBS) is above 140 mg/dL in any ICU patients.7 AI
- HbA1c should be done for all diabetic ICU patients, if it has not been done in past 2–3 months.7 AI
- If HbA1c is above 6.5% (even if RBS is less than 140 mg/dL), then the patient should be managed as a diabetic.7 AI
- 2-hour oral post-glucose (75 g) BG value of >140 mg/dL can also be used as another marker for diagnosing DG.3,5 BI
- HbA1c cut-off (>6.5%) along with random BG (or possibly fasting BG) should be used for DG diagnosis in all patients undergoing major surgeries and at-risk patients undergoing minor surgeries.4,5,8 BI
- Bedside capillary glucose values should be used for diagnosing DG. However, in hypotension/shock and/or hypoglycemia, arterial or venous BG values are more reliable.9 BI
- Once a day RBS should be done in all ICU patients. CI
| Evidences | Grade |
|---|---|
| Evidence from at least one existing guidelines, properly designed RCTs, meta-analysis, systematic reviews | A |
| Evidence from at least one well-designed clinically controlled analytic studies, time-series studies, dramatic results from uncontrolled experiments | B |
| Evidence from opinions of respected authorities based on clinical experience, advisory board faculties, descriptive studies, and expert committee reports | C |
| Consensus agreement | Grade |
|---|---|
| Agreement by >90% of advisory board faculties | I |
| Agreement by %3C;90% of advisory board faculties | II |
Monitoring/Assessment of Dysglycemic
Like hyperglycemia, GV is associated with higher mortality by increasing oxidative stress, neuronal damage, mitochondrial damage, and coagulation activity.10 It has been shown that rapid fluctuations of BG levels increase oxidative stress and are more detrimental than sustained hyperglycemia. Hence, all such variabilities that expose the patient to negative physiological consequences should be avoided by timely assessment.
In the chronic setting, apart from the use of the HbA1C and daily BG monitoring, variability in glucose control has also been proposed as an added measure of adequacy of control.11
Various randomized trials or retrospective studies have suggested that a relationship exists between GV and hospital outcomes, which is independent of hypo/hyperglycemia. A systematic review showed relationship between measures of GV and nonglycemic outcomes, including ICU/in-hospital mortality and length of stay.12
Hence, appropriate metrics should be developed to recognize recurrent large variability in oscillations of BG and to recognize any time-dependent change of the overall glycemic status during hospitalization.
Practice Guidelines
- The 4-hourly blood sample should be used for detecting GV in patients on continuous feeds.7 AI
- The arterial/venous blood sample is preferable over the capillary sample for continuous monitoring of GV.9 BI
- Standard deviation (SD) should be used as a measure of GV for all critically ill dysglycemic patients. If feasible, mean amplitude of glycemic excursions (MAGE) may be used as an additional measure.13 CI
- Time in target range (TITR) is another important parameter for measuring GV.14 BII
- Glycemic variability/fluctuation should be kept minimal during the entire ICU stay.15 CI
- Continuous glucose monitoring (CGM) is preferable in critical care settings, if resources are available.16 CI
Acceptable Glycemic Targets for Dysglycemic Patients in Intesive Care Unit
The management of DG in the hospital presents unique challenges due to variations in a patient’s nutritional status, level of consciousness, and the practical limitations of CGM. Accordingly, reasonable glucose targets proposed in the hospital setting remain modestly higher than the targets routinely advised for diabetic patients in the outpatient setting.17
Van den Berghe et al. (Leuven I trial)18 found that intensive insulin therapy lowered surgical ICU mortality from 8.0% to 4.6% (absolute risk reduction, 3.4%) and in-hospital mortality from 10.9% to 7.2% (absolute risk reduction, 3.7%). Intensive insulin therapy also reduced morbidity by preventing organ failure. These findings could be attributable to prevention of glucose toxicity to vital cells, shown in various human and animal studies.19
The Leuven II hypothesis was tested in a medical ICU setting.20 The in-hospital mortality rates were 40.0% in the control group and 37.3% in the intervention group, which were not statistically significant in an intention-to-treat analysis of the 1,200 included patients. Similar organ-protective effects were documented but not as strikingly as in the surgical study.
Later, the volume substitution and insulin therapy in severe sepsis (VISEP) multicenter trial (n = 537) and the glucontrol multicenter trial (n = 1101) also failed to reproduce Leuven I findings.18
A meta-analysis of over 26 studies, including the normoglycemia in intensive care evaluation-survival using glucose algorithm regulation (NICE-SUGAR) study, showed increased rates of “severe hypoglycemia” (defined in the analysis as BG 40 mg/dL) and higher mortality in cohorts with tight vs moderate glycemic control.21 Recent randomized controlled studies and meta-analyses in surgical patients have also reported that targeting perioperative BG levels to 180 mg/dL is associated with lower rates of mortality and stroke compared with a target glucose 200 mg/dL, whereas no significant additional benefit was found with stricter glycemic control (<140 mg/dL).22
Hence, the current literature/recommendations favor moderate glycemic control. Various guidelines recommend quite similar target/ranges for BG levels. However, permissible ranges for other parameters like HbA1c ranges, standard deviation, and BG measurement frequency with respect to various modalities of feeding are often not discussed.
Practice Guidelines
- The preferred BG range for medical/surgical ICU patients is 140–180 mg/dL.7 AI
- Frequency of BG monitoring should be seven times a day in orally fed/bolus-fed dysglycemic patients.16 AI
- Blood glucose measurements should be done 4 hourly in continuously fed dysglycemic patients.23 AI
- Monitoring and adherence of glycemic targets is mandated. However, adherence can be continued longer, if there is persistent DG or the patient is on steroids.7 AI
- Hypoglycemic episodes should be minimal, and efforts should be made for keeping BG levels above 110 mg/dL.5 AI
- The permissible range for SD is within ±1. CI
Continuous vs Bolus Feeding: Which is Better for Dysglycemic Patients
Adequate nutrition remains the key aspect of patients’ care, especially for the dysglycemic critically ill patients.24 Timely and appropriate nutritional support is found to be effective in achieving expected outcomes in ICU patients.25 Research showed that 33–92% of patients in ICU receive enteral feeding.26 Enteral feeding can be administrated through three routes of oro-/nasogastric, gastrostomy, and jejunostomy tubes. Continuous, intermittent, and bolus feeding modalities are used, depending on patients’ clinical requirements and clinician’s judgment.27
Duggan showed that continuous tube feeding was effective in the controlling BG levels through making a change in a usual pattern of insulin and glucagon secretions.28
Practice Guidelines
Glycemic Index/Glycemic Load/Carbohydrate Counting and Clinical Relevance
Diet is an important variable in prevention as well as management of DG. Initially, dietary recommendations emphasized control of the amount of carbohydrates without focusing on the carbohydrate quality. Discovery of postprandial glycemia (PPG) as an independent risk factor for cardiovascular disease resulted in clinical interest in the carbohydrate composition and quality. In early 1980s, Jenkins et al. established a standard called the “glycemic index (GI),” for classifying carbohydrates according to their effect on PPG.30
The GI is a relative ranking of carbohydrates in foods according to how they affect BG levels. Carbohydrates with a low GI value (55 or less) are more slowly digested, absorbed, and metabolized and cause a lower and slower rise in BG and, therefore usually, insulin levels. One meta-analysis of studies on GI showed that low GI feeds significantly improved glycemic control.31 Low GI is also found to favorably influence the lipid profile. Low GI feeds also correlate well with improved insulin sensitivity, improved fibrinolytic activity, and decreased chronic inflammation.32 Low GI mildly increases the benefits over a diet based on mere carbohydrate quantity intake calculation.33
The GI is a measure of quality of carbohydrates. Two parameters reflecting quantity of carbohydrates are glycemic load (GL) and carbohydrate counting (CC). Glycemic load is adjusted for available carbohydrate and correlates well with the overall glycemic effect of a diet.34,35 Glycemic load combines both the quantity and quality of carbohydrates. It is also the best way to compare BG values of several types and amounts of foods. Glycemic load is calculated by multiplying the grams of available carbohydrate in the food with its GI and dividing by hundred. The achieved values are classified as high, medium, and low for %3E;20, 11–19, and <10, respectively.36 High GL is also found associated with the risk of hyperglycemia in type II diabetic patients.37 Hence, GI and GL should be viewed in conjunction and need to be an important aspect of any glycemia-targeted specialized nutrition (GTSN) feed.
Carbohydrate counting is an established approach used by patients with type I diabetes to improve their glycemic control. Patients, thus, over- or underestimate carbohydrate amounts, which results in hypo- or hyperglycemia. One study found inaccurate CC as frequent and associated with higher daily BG variability.38 Hence, CC needs to be made more precise.
Practice Guidelines
- Glycemia-targeted specialized nutrition should be identified by its low GI/GL rather than individual carbohydrate ingredients alone.39,40 AI
- The GI and GL are precisely known for scientifically formulated/formula feeds; however, their estimation is not precise for kitchen-made feeds.41,42 AI
- Low GI/GL is an important parameter with regards to feeds for dysglycemic patients.39,40,43 BI
Glycemia-targeted Specialized Nutrition
Proteins, fats, and carbohydrates are the three main categories of macronutrients and providers of energy. An appropriate proportion of these three macronutrient categories is essential to maintain caloric adequacy, protein sparing, and overall balance in nutrition. For patients requiring a controlled glucose level and nutrition support, GTSN feeds/formulae that contain slowly digestible carbohydrates and monounsaturated fatty acids (MUFAs) should be used. A growing body of evidence has already suggested that such feeds/formulae are associated with improved glycemic control and improved insulin resistance as compared to a standard nutrition formula in diabetic patients.44,45 Such feeds/formulae have also been shown to improve glycemic control in critically ill ICU patients.46
Mesejo et al.47 found better PPG parameters when diabetes-specific nutrition feeds were used in mechanically ventilated critically ill patients,46 indicating that diabetes-specific feeds can also be used as GTSN for nutritional management of DG in ICU patients. Shao et al. found similar benefits, when stroke patients were put on enteral diabetes-specific nutrition feeds.48
To evaluate whether low-carbohydrate feeds alone could qualify to be effectively used as GTSN, van Steen et al. did an open-label RCT. They found modestly reduced mean glucose and significantly lower insulin requirements with such feeds as compared with standard feeding. However, no favorable effect was seen on GV parameters, thus questioning whether low-carbohydrate feeds alone could be used as GTSN.49
The benefits of such GTSN feeds/formulae are primarily due to their unique blends of amino acids, complex carbohydrates, and fats and are achieved by either directly stimulating insulin secretion or stimulating glucagon-like peptide (GLP)-1 secretion.42
Practice Guidelines
- Purely low-carbohydrate feeds may not reduce postprandial GV.49 AI
- The protein and electrolyte content of a GTSN should be taken in consideration in renal/hepatic patients with fluid/electrolyte imbalance.50,51 AI
- Glycemia-targeted specialized nutrition low in GI/GL and containing optimal protein is important for nutritional management of dysglycemic critically ill patients (whether diabetic52 BI or nondiabetic DG47,48 BI)
- Feeds containing complex/slowly digestible carbohydrates and MUFA help in glycemic optimization in critical care settings.42,47,48,52 BI
Macronutrients and Glucagon-like Peptide-1 Modulation
Traditionally, carbohydrates were considered the predominant macronutrient affecting postprandial glucose control. Recent studies have shown that other nutritional properties of food including fat, protein, and GI can also significantly affect PPG controls.
Proteins could stimulate GLP-1 release even more than carbohydrates. It was found that milk proteins with higher proportion of leucine and isoleucine potently stimulated GLP-1 secretion.53
Nondigestible and fermentable carbohydrates (prebiotics), as well as short-chain fatty acids (SCFAs), have been shown to increase GLP-1 levels.54 Short-chain fatty acids are produced by bacterial fermentation of nondigestible carbohydrates. Apart from increasing GLP-1 secretion, SCFAs also act as local nutrients for colonic mucosal cells.
Devitt et al.42 and Voss et al.52 concluded that the formula rich in slowly digested complex carbohydrates and MUFAs produced significantly lower BG and insulin responses and higher levels of GLP-1 in the presence of significantly lower insulin concentrations.
High protein digestibility-corrected amino acid score (PDCAAS) proteins along with complex carbohydrates and MUFAs have tendency to favorably modulate the GLP-1 response and hence minimize GV.52–54
Practice Guidelines
Specialized Cases (Cardiac, Renal, Neurological Cases) and DG Nutrition
Chronic hyperglycemia, which hallmarks diabetes also, predisposes to vascular complications.56 Microvascular changes cause blindness, renal dysfunction, and nerve damage, whereas macrovascular atherosclerotic damage increases the risk of stroke, myocardial infarction (MI), and limb amputation.
Cardiac Patients
Independent of the patient’s diabetic status, DG is frequent in patients undergoing cardiac surgical procedures. Perioperative DG is found to be associated with poor outcomes in acute MI patients both with and without diabetes. Chronic glucose dysregulation, as assessed by HbA1c levels, is a prognostic factor for mortality in acute MI (AMI) patients.57 Admission hyperglycemia is found to be an independent prognostic factor regarding future adverse cardiovascular events in such patients.58
Su et al. found early in-hospital intraday glycemic excursion as an important predictor of mortality and the major adverse cardiac event (MACE), even stronger than HbA1c in elderly patients after acute MI.59 Nam et al. found intraoperative glucose variability, but not the average glucose concentration itself, as a risk factor for acute kidney injury (AKI) after cardiac surgery.60 The impact of admission hyperglycemia on development of AKI in patients with AMI was found by Moriyama et al.61
Hence, a fair amount of literature exists, discussing the significance of glycemic management in cardiac critically ill patients and the complications arising out of episodes of DG. However, when it comes to nutrition intervention for managing DG, the literature is scarce.
Practice guidelines
- The preferred BG range for medical/surgical ICU patients is 140–180 mg/dL.7 AI
- Hypoglycemic episodes should be minimal, and efforts should be made for keeping blood sugar levels above 110 mg/dL.5 AI
- If HbA1c is above 6.5% (even if RBS is less than 140), then patients should be managed as a diabetic.7 AI
- Frequency of BG monitoring should be seven times a day in orally fed/bolus-fed dysglycemic patients.16 AI
- Blood glucose measurements should be done 4 hourly in continuous-fed dysglycemic patients.23 AI
- Glycemia-targeted specialized nutrition can be administered to cardiac ICU patients, to favorably manage GV.62 BI
Neurological Patients
Dysglycemia induces injury to vulnerable areas of the nervous system and contributes to the development of neurologic complications including delirium, polyneuropathy, and long-term cognitive impairment in ICU survivors.63 Interestingly, the onset of delirium is characterized by an acute brain dysfunction involving the hippocampus and the frontal cortex. These two areas are extremely vulnerable to metabolic insults like hypoxia, hypoglycemia, and possibly hyperglycemia.64
Hyperglycemia is frequently observed in patients with acute brain injury.65 In cerebral ischemia, hyperglycemia has various deleterious effects, including increased infarct volume, impaired recanalization, and decreased reperfusion. This damage is caused by reperfusion injury and direct tissue injury.66
Reducing GV, irrespective of BG concentration, may produce clinical benefits, including neuroprotection. This is achieved by reducing glucose reperfusion injury. Various existing guidelines elaborately discuss the meticulous DG management for such patients by pharmacological intervention. However, its nutritional angle is ignored by most of them.
Practice guidelines
- The preferred BG range for medical/surgical ICU patients is 140–180 mg/dL.7 AI
- Hypoglycemic episodes should be minimal, and efforts should be made for keeping blood sugars levels above 110 mg/dL.5 AI
- If HbA1c is above 6.5% (even if RBS is less than 140), then patients should be managed as diabetic.7 AI
- Frequency of BG monitoring should be seven times a day in orally fed/bolus-fed dysglycemic patients.16 AI
- Blood glucose measurements should be done 4 hourly in continuous fed dysglycemic patients.23 AI
- Glycemia-targeted specialized nutrition can be administered to neuro ICU patients, to favorably manage GV.47 BI
Renal/Hepatic Patients
Acute kidney injury is a frequent complication after major surgeries.67 Intraoperative hyperglycemia and high GV have been identified as independent risk factors for renal dysfunction after surgery.68
Acute loss of renal function interferes with the metabolism of all macronutrients. It is also responsible for proinflammatory, pro-oxidative, and hypercatabolic situations. Hypercatabolism, hyperglycemia, and hypertriglyceridemia are major nutritional disorders in AKI patients. Dialysis causes further nutritional depletion in such patients. Ikizler et al. found that dialysis was responsible for a 133% increase in muscle protein degradation and sustained degradation of total body protein even after the end of the dialysis.69
In a prospective observation study, Marezi et al. found that in diabetic patients with AMI, AKI is better predicted by the combined evaluation of acute and chronic glycemic values than by assessment of admission glycemia alone.70 Clear correlation between preoperative hyperglycemia (>300 mg/dL) and postoperative AKI was found in a prospective observational multicenter study of 2,222 patients undergoing coronary artery bypass graft (CABG) surgery by Mangano et al.71
Other studies found elevated preoperative HbA1c >6% consistently associated with a higher incidence of postoperative AKI.72 Similar pathophysiological changes occur in hepatic involvement in critical care settings. Dysglycemia is harmful in such patients as well.
Hence, it becomes very important to manage DG in critically ill patients suffering with concomitant renal/hepatic ailments, both pharmacologically and nutritionally. European Society of Enteral and Parenteral Nutrition( ESPEN)51 and kidney disease outcome and quality initiative (KDOQI)52 guidelines have elaborately discussed nutrition care for such patients. However, a specific focus on nutrition management of DG in such patients is not clarified.
Practice guidelines
- The preferred BG range for medical/surgical ICU patients is 140–180 mg/dL.7 AI
- Hypoglycemic episodes should be minimal, and efforts should be made for keeping blood sugar levels above 110 mg/dL.5 AI
- If HbA1c is above 6.5% (even if RBS is less than 140), then patients should be managed as diabetic.7 AI
- Frequency of BG monitoring should be seven times a day in orally fed/bolus-fed dysglycemic patient.16 AI
- Blood glucose measurements should be done 4 hourly in continuous fed dysglycemic patients.23 AI
- A renal-specific feed, low in GI/GL and containing optimal proteins/electrolytes, can be used for nutrition support in renal ICU patients with specific protein requirements and/or water/electrolyte imbalances.1 AI
- In such patients requiring dialysis, due consideration should be given to their protein/electrolyte requirements, while choosing renal-specific feeds.51 AI
- Glycemia-targeted specialized nutrition can be administered to such ICU patients (without specific protein requirements and no water/electrolyte imbalances), to favorably manage GV.50 CI
Team Effort in Dysglycemic Management
From screening of patients for DG, prediction of subsequent DG, regular assessment of the glycemic status, and glycemic control by pharmacotherapy or nutrition intervention requires an efficient team effort. A collaborative team work of healthcare practitioners, viz., doctors, nutritionists, and paramedics, is very important. Ranging from documentation to raising red flags and active management of DG ultimately results in better prognosis.
Ginde et al.73 reported that only 10% of patients identified as hyperglycemic in emergency departments had hyperglycemia communicated to the attending team, and only 6% had documented plans in the medical chart for further evaluation and management of DG. Recognition, communication, and management of hyperglycemia were suboptimal. It represented a missed opportunity for identifying undiagnosed diabetes and initiating early glycemic control for such patients.
Tonks et al.74 found equally low rates of written communication of DG by care providers in a large retrospective review of patients with previously undiagnosed diabetes. They concluded that changes should be made in systems for checking pathology results, medical officer education, and inpatient screening protocols.
Practice Guidelines
- Define hospital-specific protocols for delineating roles of support staff (nutritionists, nurses, pharmacists) in managing DG.23,75 BI (details mentioned in Annexure I)
- Defining a hospital-specific protocol for proper communication of DG to the patient and family is important.23,75 BI
- Regular glycemia management training sessions, requiring at least 6 hours of mandatory training per year for healthcare providers. CI
- Regular nutrition management training sessions, requiring at least 6 hours of mandatory training per year for healthcare providers. CI
Nutrition in Transition (Patient Shifting from ICU to Ward)
A structured and tailored discharge plan will help to reduce length of hospital stay and readmission rates.23 Facilitating safer transition from critical care settings would require clear and effective communication with in-/outpatient healthcare providers. Information regarding the cause of hyperglycemia (or the plan for determining the cause), related complications/comorbidities, and recommended treatments should be provided during the patient hand over. Hence, in such cases, a medication reconciliation plan and structured communication become mandatory.
There are not many established recommendations regarding the acceptable ranges of glycemic control as well as frequency of monitoring, except mentioned by Research Society for the Study of Diabetes in India (RSSDI) guidelines 2016.7 Also, the literature discussing the role of GTSN in patients in transition is very scarce.
Practice Guidelines
- Acceptable glycemic values in hospitalized patients should be 140–180 mg/dL.7 AI
- RBS/PPG monitoring should be preferably done four times a day.14 AI. If not feasible, then as deemed appropriate by the treating physician. CI
- Glycemia-targeted specialized nutrition can be used in all such patients who were dysglycemic in their ICU stay and continued till the patient is hospitalized.39,43 BI
- Patients with chronic comorbidities like cardiovascular, neural, hepatic, and renal should be classified as the high-risk group. CI
- Effective hand over and documentation for such high-risk groups should be provided from ICU to the ward, ensuring continuum of care. CI
Algorithm for Nutrition Management in Dysglycemic Critically Ill Patients
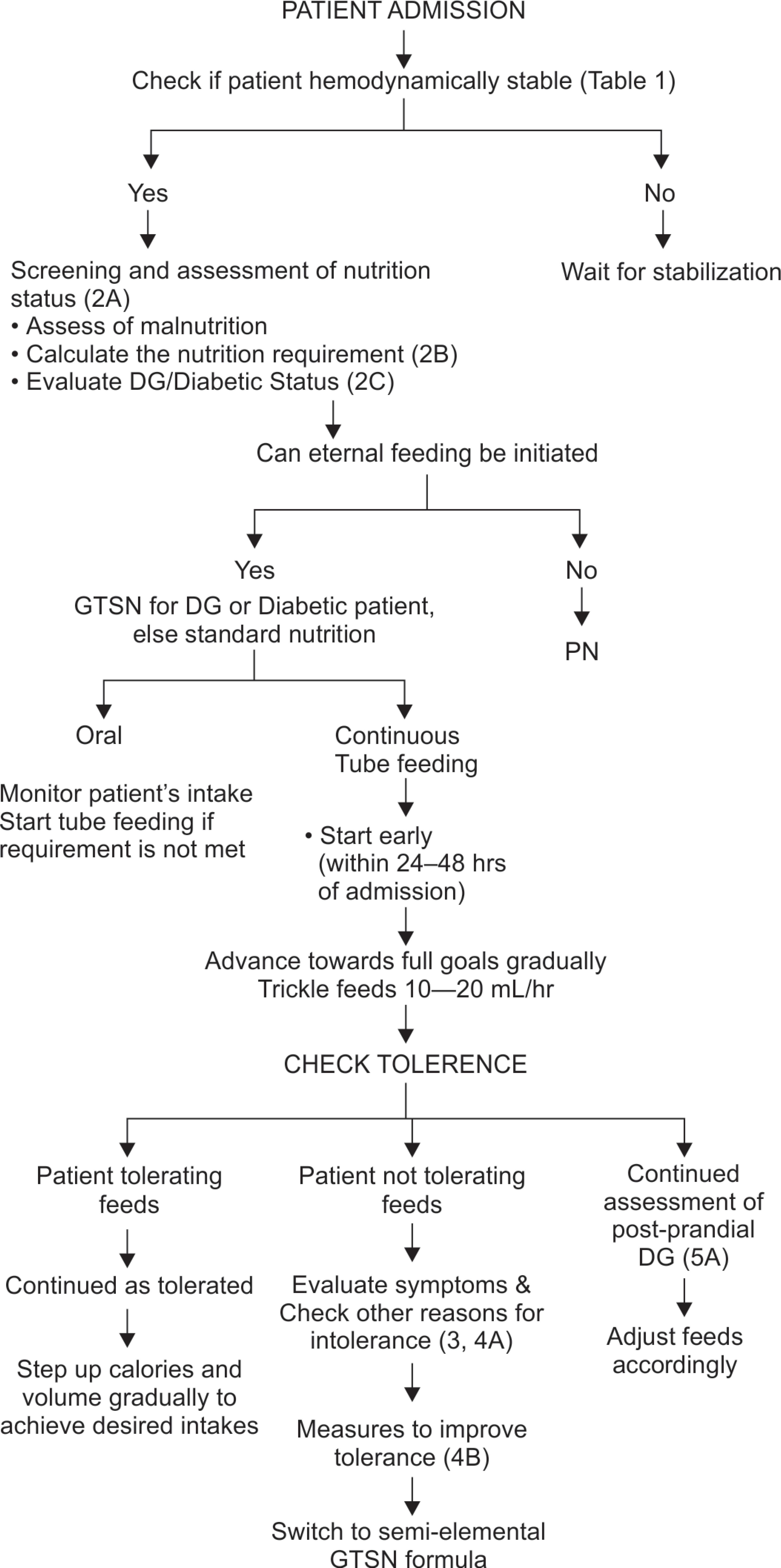
Based on above guidelines agreed upon by the experts, the algorithm published by Mehta et al.1 is accordingly modified and recommendations for DG prediction, nutrition intervention, and monitoring are included (Annexure II).
REFERENCES
1. Mehta Y, Sunavala JD, Zirpe K, Tyagi N, Garg S, Sinha S, et al. Practice guidelines for nutrition in critically ill patients: a relook for Indian scenario. Indian J Crit Care Med 2018;22(4):263–273. DOI: 10.4103/ijccm.IJCCM_3_18.
2. Janssen PG, Gorter KJ, Stolk RP, Rutten GE. Low yield of population-based screening for type 2 diabetes in the Netherlands: the ADDITION Netherlands study. Fam Pract 2007;24(6):555–561. DOI: 10.1093/fampra/cmm052.
3. Malcolm JC, Kocourek J, Keely E, Feibel RJ, Brez S, Forster AJ, et al. Implementation of a screening program to detect previously undiagnosed dysglycemia in hospitalized patients. Can J Diabetes 2014;38(2):79–84. DOI: 10.1016/j.jcjd.2014.02.005.
4. Tekumit H, Cenal AR, Polat A, Uzun K, Tataroglu C, Akinci E. Diagnostic value of hemoglobin A1c and fasting plasma glucose levels in coronary artery bypass grafting patients with undiagnosed diabetes mellitus. Ann Thorac Surg 2010;89(5):1482–1487. DOI: 10.1016/j.athoracsur.2009.11.033.
5. Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycaemic Control. Diabetes Care 2009;32(6):1119–1131. DOI: 10.2337/dc09-9029.
6. Dhatariya K, Levy N, Kilvert A, Watson B, Cousins D, Flanagan D, et al. NHS diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med 2012;29(4):420–433. DOI: 10.1111/j.1464-5491.2012.03582.x.
7. Panikar V, Sosale A, Agarwal S, Unnikrishnan A, Kalra S, Bhattacharya A, et al. RSSDI clinical practice recommendations for management of in-hospital hyperglycaemia—2016. Int J Diabetes Dev Ctries 2016;36 (Suppl 1):S1–S21. DOI: 10.1007/s13410-016-0528-z.
8. Jehan F, Khan M, Sakran JV, Khreiss M, O’Keeffe T, Chi A, et al. Perioperative glycemic control and postoperative complications in patients undergoing emergency general surgery: what is the role of Plasma Hemoglobin A1c? J Trauma Acute Care Surg 2018;84(1):112–117. DOI: 10.1097/TA.0000000000001724.
9. Finkielman JD, Oyen LJ, Afessa B. Agreement between bedside blood and plasma glucose measurement in the ICU setting. Chest 2005;127(5):1749–1751. DOI: 10.1378/chest.127.5.1749.
10. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycaemia in patients with type 2 diabetes. JAMA 2006;295(14):1681–1687. DOI: 10.1001/jama.295.14.1681.
11. Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol 2008;2(6):1094–1100. DOI: 10.1177/193229680800200618.
12. Braithwaite SS. Glycemic variability in hospitalized patients: choosing metrics while awaiting the evidence. Curr Diab Rep 2013;13(1):138–154. DOI: 10.1007/s11892-012-0345-9.
13. Eslami S, Taherzadeh Z, Schultz MJ, Abu-Hanna A. Glucose variability measures and their effect on mortality: a systematic review. Intensive Care Med 2011;37(4):583–593. DOI: 10.1007/s00134-010-2129-5.
14. Krinsley JS, Preiser JC. Time in blood glucose range 70 to 140 mg/dl >80% is strongly associated with increased survival in non-diabetic critically ill adults. Crit Care 2015;19:179. DOI: 10.1186/s13054-015-0908-7.
15. Sim MA, Liu W, Chew STH, Ti LK. Wider perioperative glycaemic fluctuations increase risk of postoperative atrial fibrillation and ICU length of stay. PLoS One 2018;13(6): e0198533. DOI: 10.1371/journal.pone.0198533.
16. Rao PV, Makkar BM, Kumar A, Das AK, Singh AK, Mithal A, et al. RSSDI consensus on self-monitoring of blood glucose in types 1 and 2 diabetes mellitus in India. Int J Diabetes Dev Countries 2018;38(3):260–279. DOI: 10.1007/s13410-018-0677-3.
17. Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007;13 (Suppl 1):1–68. DOI: 10.4158/EP.13.S1.1.
18. Van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R, et al. Clinical review: intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab 2009;94(9):3163–3170. DOI: 10.1210/jc.2009-0663.
19. Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest 2004;114(9):1187–1195. DOI: 10.1172/JCI23506.
20. Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354(5):449–461. DOI: 10.1056/NEJMoa052521.
21. NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY, Blair D, Foster D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360(13):1283–1297. DOI: 10.1056/NEJMoa0810625.
22. Sathya B, Davis R, Taveira T, Whitlatch H, Wu W-C. Intensity of peri-operative glycemic control and postoperative outcomes in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2013;102(1):8–15. DOI: 10.1016/j.diabres.2013.05.003.
23. American Diabetes Association. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42 (Suppl 1):S173–S181. DOI: 10.2337/dc19-S015.
24. Kozier B, Eeb G, Berman A, Burkc K. Fundamentals of Nursing-Concepts, Process and Practice,New York, Upper Saddle River: Prentice-Hall; 2000.
25. Stefanogiannis N, Lawes CM, Turley M, Tobias M, Hoorn SV, Mhurchu CN, et al. Nutrition and the burden of disease in New Zealand: 1997–2011. Public Health Nutr J 2005;8(4):395–401. DOI: 10.1079/PHN2004694.
26. Heyland DK, Schroter-Noppe D, Drover J, Jain M, Keefe L, Dhalwal R, et al. Nutrition support in the critical care setting: current practice in Canadian ICUs–opportunities for improvement? JPEN J Parenter Enteral Nutr 2003;27(1):74–83. DOI: 10.1177/014860710302700174.
27. Rolandelli RH. Clinical Nutrition: Enteral and tube feeding,Philadelphia: Elseviers Sanders; 2005; p. 244.
28. Duggan CH, Watkins J, Walkers W. Nutrition in pediatrics basic science. Clinical Application,Hamilton, Ontario: BC Decker Inc.; 2008.
29. Shahriari M, Rezaei E, Bakht LA, Abbasi S. Comparison of the effects of enteral feeding through the bolus and continuous methods on blood sugar and prealbumin levels in ICU inpatients. J Educ Health Promot 2015;4:95. DOI: 10.4103/2277-9531.171809.
30. Jenkins DJA, Wolever TMS, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycaemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34(3):362–366. DOI: 10.1093/ajcn/34.3.362.
31. Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes. Diabetes Care 2003;26(8):2261–2267. DOI: 10.2337/diacare.26.8.2261.
32. Levitan EB, Cook NR, Stampfer MJ, Ridker PM, Rexrode KM, Buring JE, et al. Dietary glycaemic index, dietary glycaemic load, blood lipids, and C-reactive protein. Metabolism 2008;57(3):437–443. DOI: 10.1016/j.metabol.2007.11.002.
33. American Diabetes Association. Nutrition principles and recommendations in diabetes. Diabetes Care 2008;31 (Suppl 1):S61–S78. DOI: 10.2337/dc08-S061.
34. Jenkins DJA, Kendall CW, Augustin LS, Franceschi S, Hamidi M, Marchie A, et al. Glycemic index: overview of implications in health and disease. Am J Clin Nutr 2002;76(1):266S–273S. DOI: 10.1093/ajcn/76.1.266S.
35. Sieri S, Brighenti F, Agnoli C, Grioni S, Masala G, Bendinelli B, et al. Dietary glycemic load and glycemic index and risk of cerebrovascular disease in the EPICOR cohort. PLoS One 2013;8(5): e62625. DOI: 10.1371/journal.pone.0062625.
36. https://www.gisymbol.com/about-glycemic-index/ ,accessed on 19-08-2019.
37. Sieri S, Krogh V, Berrino F, Evangelista A, Agnoli C, Brighenti F, et al. Dietary glycaemic load and index and risk of coronary heart disease in a large Italian cohort: the EPICOR study. Arch Intern Med 2010;170(7):640–647. DOI: 10.1001/archinternmed.2010.15.
38. Farvid MS, Homayouni F, Shokoohi M, Fallah A, Farvid MS. Glycemic index, glycemic load and their association with glycemic control among patients with type 2 diabetes. Eur J Clin Nutr 2014;68(4):459–463. DOI: 10.1038/ejcn.2013.288.
39. Alish CJ, Garvey WT, Maki KC, Sacks GS, Hustead DS, Hegazi RA, et al. A diabetes-specific enteral formula improves glycemic variability in patients with type 2 diabetes. Diabetes Technol Ther 2010;12(6):419–425. DOI: 10.1089/dia.2009.0185.
40. Visek J, Lacigova S, Cechurova D, Rusavy Z. Comparison of a low-glycaemic index vs standard diabetic diet. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014;158(1):112–116. DOI: 10.5507/bp.2012.103.
41. Ojo O, Ojo OO, Adebowale F, Wang XH. The effect of dietary glycaemic index on glycaemia in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients 2018;10(3):E373. DOI: 10.3390/nu10030373.
42. Devitt AA, Oliver JS, Hegazi RA, Mustad VA. Glycemia targeted specialized nutrition (GTSN) improves postprandial glycemia and GLP-1 with similar appetitive responses compared to a healthful whole food breakfast in persons with type 2 diabetes: a randomized, controlled trial. J Diabetes Res Clin Metab 2012;1:20. DOI: 10.7243/2050-0866-1-20.
43. Brazeu AS, Mircescu H, Desjardins K, Leroux C, Strychar I, Ekoé JM, et al. Carbohydrate counting accuracy and blood glucose variability in adults with type 1 diabetes. Diabetes Res Clin Pract 2013;99(1):19–23. DOI: 10.1016/j.diabres.2012.10.024.
44. Mottalib A, Mohd-Yusof BN, Shehabeldin M, Pober DM, Mitri J, Hamdy O. Impact of diabetes-specific nutritional formulas versus oatmeal on postprandial glucose, insulin, GLP-1 and postprandial lipidemia. Nutrients 2016;8(7):E443. DOI: 10.3390/nu8070443.
45. Vaisman N, Lansink M, Rouws CH, van Laere KM, Segal R, Niv E, et al. Tube feeding with a diabetes-specific feed for 12 weeks improves glycaemic control in type 2 diabetes patients. Clin Nutr 2009;28(5):549–555. DOI: 10.1016/j.clnu.2009.05.004.
46. Pietraszek A, Gregersen S, Pedersen SB, Holst JJ, Hermansen K. Acute effects of monounsaturated fat on postprandial lipemia and gene expression in first-degree relatives of subjects with type 2 diabetes. Eur J Clin Nutr 2014;68(9):1022–1028. DOI: 10.1038/ejcn.2014.64.
47. Mesejo A, Montejo-Gonz´alez JC, Vaquerizo-Alonso C, Lobo-Tamer G, Zabarte-Martinez M, Herrero-Meseguer JI, et al. Diabetes-specific enteral nutrition formula in hyperglycemic, mechanically ventilated, critically ill patients: a prospective, open-label, blind-randomized, multicenter study. Crit Care 2015;19:390. DOI: 10.1186/s13054-015-1108-1.
48. Shao Y, Heng W, Li S, Xu Y, Hu G. Tube feeding with a diabetes-specific enteral formula improves glycemic control in severe acute ischemic stroke patients. JPEN J Parenter Enteral Nutr 2018;42(5):926–932. DOI: 10.1002/jpen.1035.
49. van Steen SC, Rijkenberg S, Sechterberger MK, DeVries JH, van der Voort PHJ. Glycemic effects of a low-carbohydrate enteral formula compared with an enteral formula of standard composition in critically Ill patients: an open-label randomized controlled clinical trial. JPEN J Parenter Enteral Nutr 2017;42(6):1035–1045. DOI: 10.1002/jpen.1045.
50. Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr 2019;38(2):485–521. DOI: 10.1016/j.clnu.2018.12.022.
51. KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 2007;49(2 Suppl 2):S12–S154. DOI: 10.1053/j.ajkd.2006.12.005.
52. Voss AC, Maki KC, Garvey WT, Hustead DS, Alish C, Fix B, et al. Effect of two carbohydrate-modified tube-feeding formulas on metabolic responses in patients with type 2 diabetes. Nutrition 2008;24(10):990–997. DOI: 10.1016/j.nut.2008.06.009.
53. Fekete ÁA, Givens DI, Lovegrove JA. Can milk proteins be a useful tool in the management of cardiometabolic health? An updated review of human intervention trials. Proc Nutr Soc 2016;75(3):328–341. DOI: 10.1017/S0029665116000264.
54. Mansour A, Hosseini S, Larijani B, Pajouhi M, Mohajeri-Tehrani MR. Nutrients related to GLP1 secretory responses. Nutrition 2013;29(6):813–820. DOI: 10.1016/j.nut.2012.11.015.
55. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012;61(2):364–371. DOI: 10.2337/db11-1019.
56. The Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329(14):977–986. DOI: 10.1056/NEJM199309303291401.
57. Cakmak M, Cakmak N, Cetemen S, Tanriverdi H, Enc Y, Teskin O, et al. The value of admission glycosylated haemoglobin level in patients with acute myocardial infarction. Can J Cardiol 2008;24(5):375–378. DOI: 10.1016/s0828-282x(08)70600-7.
58. Stranders I, Diamant M, van Gelder RE, Spruijt HJ, Twisk JW, Heine RJ, et al. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med 2004;164(9):982–988. DOI: 10.1001/archinte.164.9.982.
59. Su G, Mi SH, Li Z, Tao H, Yang HX, Zheng H. Prognostic value of early in-hospital glycemic excursion in elderly patients with acute myocardial infarction. Cardiovasc Diabetol 2013;12:33. DOI: 10.1186/1475-2840-12-33.
60. Nam K, Jeon Y, Kim WH, Jung DE, Kwon SM, Kang P, et al. Intraoperative glucose variability, but not average glucose concentration, may be a risk factor for acute kidney injury after cardiac surgery: a retrospective study. Can J Anesth 2019;66(8):921–933. DOI: 10.1007/s12630-019-01349-0.
61. Moriyama N, Ishihara M, Noguchi T, Nakanishi M, Arakawa T, Asaumi Y, et al. Admission hyperglycaemia is an independent predictor of acute kidney injury in patients with acute myocardial infarction. Circ J 2014;78(6):1475–1480. DOI: 10.1253/circj.cj-14-0117.
62. de Azevedo JR, de Araujo LO, da Silva WS, de Azevedo RP. A carbohydrate-restrictive strategy is safer and as efficient as intensive insulin therapy in critically ill patients. J Crit Care 20010;25(1):84–89. DOI: 10.1016/j.jcrc.2008.10.011.
63. Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE,Jr et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291(14):1753–1762. DOI: 10.1001/jama.291.14.1753.
64. Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, et al. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet 2003;362(9398):1799–1805. DOI: 10.1016/s0140-6736(03)14899-4.
65. Ntaios G, Egli M, Faouzi M, Michel P. J-shaped association between serum glucose and functional outcome in acute ischemic stroke. Stroke 2010;41(10):2366–2370. DOI: 10.1161/STROKEAHA.110.592170.
66. Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycaemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol 2010;6(3):145–155. DOI: 10.1038/nrneurol.2009.231.
67. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120(4):c179–c184. DOI: 10.1159/000339789.
68. Song JW, Shim JK, Yoo KJ, Oh SY, Kwak YL. Impact of intraoperative hyperglycaemia on renal dysfunction after off-pump coronary artery bypass. Interact Cardiovasc Thorac Surg 2013;17(3):473–478. DOI: 10.1093/icvts/ivt209.
69. Ikizler TA, Pupim LB, Brouillette JR, Levenhagen DK, Farmer K, Hakim RM, et al. Haemodialysis stimulates muscle and whole-body protein loss and alters substrate oxidation. Am J Physiol Endocrinol Metab 2002;282(1):E107–E116. DOI: 10.1152/ajpendo.2002.282.1.E107.
70. Marezi G, Cosentino N, Milazzo V, De Metrio M, Rubino M, Campodonico J, et al. Acute kidney injury in diabetic patients with acute myocardial infarction: role of acute and chronic glycemia. J Am Heart Assoc 2018;7(8): e008122. DOI: 10.1161/JAHA.117.008122.
71. Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. the multicentre study of perioperative ischemia research group. Ann Intern Med 1998;128(3):194–203. DOI: 10.7326/0003-4819-128-3-199802010-00005.
72. Halkos ME, Lattouf OM, Puskas JD, Kilgo P, Cooper WA, Morris CD, et al. Elevated preoperative hemoglobin A1c level is associated with reduced long-term survival after coronary artery bypass surgery. Ann Thorac Surg 2008;86(5):1431–1437. DOI: 10.1016/j.athoracsur.2008.06.078.
73. Ginde AA, Savaser DJ, Camargo CA.Jr Limited communication and management of emergency department hyperglycaemia in hospitalized patients. J Hosp Med 2009;4(1):45–49. DOI: 10.1002/jhm.400.
74. Tonks KT, Jones GR, McGeechan K, Campbell LV. Hyperglycaemia in hospital inpatients: still a sticky situation. Intern Med J 2010;40(7):521–526. DOI: 10.1111/j.1445-5994.2010.02197.x.
75. Amrith BP, Sethi P, Soneja M, Vikram N, Kumar A, Aggarwal P, et al. Effect of implementation of ADA/AACE guidelines on the management of hospitalized hyperglycaemic patients through training of residents: a tertiary care centre study. Indian J Endocr Metab 2018;22(5):616–620. DOI: 10.4103/ijem.IJEM_698_17.
ANNEXURE I
Glycemia Control Task Force
The glycemia control task force should be constituted in all the critical care settings, under the supervision of the head of the intensive care department. It should include physicians/intensivists, clinical nutritionists, and nurses. The primary responsibilities of the glycemia control task force members should be as follows:
- For nurses:
- Universal precautions for maintaining feed and delivery hygiene
- Knowledge of enteral feeding techniques in general and continuous enteral tube feeding in particular
- Feed interruption/modification to be done only after written instructions from physicians/clinical nutritionists
- Monitoring of BG and adherence to the hospital protocol
- Monitor signs of feed intolerance for reporting to the physician/clinical nutritionist
- Documentation of nutrition care
- For clinical nutritionists:
- Nutritionist rounds should accompany physician rounds
- Planning nutrition intake and the relevant GTSN feed for the patients based on the glycemic status in consultation with the physician
- Instructing feeding rates to nurses, monitoring nutrient delivery and feed tolerance
- Reporting nutrient inadequacy, electrolyte imbalances, feed intolerance, and related complications to the physician
- Revising nutrition intake periodically (at least once a day), based on the patient’s clinical and DG status
- Documentation of nutrition care
- For physicians/intensivists:
- Recognizing and screening of high-risk patients for DG on admission
- Utilizing nutrition intervention with pharmacological therapy to maintain the euglycemic status in DG patients in consultation with an endocrinologist (if available)
- Decision to initiate and interrupt enteral feeding, on the basis of patient’s hemodynamic stability and glycemic condition, along with the nutritionist
- Decision to switch from the parenteral to the enteral mode of nutrition and vice versa
- Management of electrolyte imbalances, water balance, and reported feed intolerances
- Documentation of nutrition care
- Ensuring adherence of team members to DG protocols of hospitals
ANNEXURE II
________________________
© The Author(s). 2019 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.