CASE REPORT | https://doi.org/10.5005/jp-journals-10071-23351 |
Case of Near Fatal Massive Intracerebral Bleed Secondary to Cerebral Venous Thrombosis in a Patient with Dengue and Refractory Thrombocytopenia
1–4Department of Critical Care Medicine, Fortis Hospital, Bengaluru, Karnataka, India
Corresponding Author: Megha Sharma, Department of Critical Care Medicine, Fortis Hospital, Bengaluru, Karnataka, India, Phone: +91 9845137934, e-mail: dr.sharmamegha@yahoo.com
How to cite this article Sharma M, Chandan GS, Arayamparambil PV, Gopalakrishna UK. Case of Near Fatal Massive Intracerebral Bleed Secondary to Cerebral Venous Thrombosis in a Patient with Dengue and Refractory Thrombocytopenia. Indian J Crit Care Med 2020; 24(2):138–140.
Source of support: Nil
Conflict of interest: None
ABSTRACT
We present a case of dengue with refractory thrombocytopenia who developed cerebral venous thrombosis (CVT) with intraparenchymal hemorrhage warranting surgical decompression. Patient was concluded to have secondary immune thrombocytopenic purpura (ITP) which remained refractory to high dose steroids, IVIg therapy, but responded to thrombopoietin receptor (TPO-R) agonist, eltrombopag.
Keywords: Cerebral venous thrombosis, Dengue, Eltrombopag, Thrombocytopenia.
CASE DESCRIPTION
We present to you a case of a 30-year-old female who was admitted to our hospital with complaints of fever and was diagnosed to have dengue. Her platelet count was 55,000/mm3 on admission. The patient was started on fluid resuscitation. On the eighth day of admission, patient started complaining of severe headache with worsening sensorium and new onset right sided hemiparesis. MRI brain with contrast was done which showed large acute left temporal hematoma with mass effect with features of cerebral venous thrombosis (CVT) involving left transverse sinus, sigmoid sinus and left internal jugular vein.
She was managed with continued fluid resuscitation. Thrombocheck panel, an antinuclear antibody test was done to look for any predisposing prothrombotic factors. Anticoagulation could not be considered in view of severe thrombocytopenia. She required repeated platelet transfusions to keep platelet count above 50,000/mm3. On 11th day patient deteriorated neurologically, with CT brain showing an increase in mass effect and midline shift.
She underwent left front temporoparietal decompressive craniectomy. Because of refractory thrombocytopenia, she was started on dexamethasone 40 mg/day. Follow up CT brain showed acute hematoma in the left temporal occipital lobe with perilesional edema and mass effect (Fig. 1). Thrombocheck panel, LDH, peripheral smear, ADAM TS 13, C3 C4 were done to rule out secondary causes of thrombocytopenia which were normal. Eltrombopag and high dose methylprednisolone (1 g/kg × 3 days) was started because of refractory thrombocytopenia with intracranial hematoma, however, her platelets continued to be low. Bone marrow biopsy showed a normocellular marrow. IVIg 1 g/kg was given as a single dose on day 17 as her platelet trends didn’t show any improvement. After 23rd day patient’s platelet count showed an increasing trend and she was taken for a relook craniotomy and hematoma evacuation. Therapeutic anticoagulation was started after 72 hours of surgery. Eltrombopag was stopped subsequently. The patient improved neurologically.
DISCUSSION
Dengue can be present as a wide range of clinical phenotypes.
Although, hemorrhagic complications are more common in dengue, thrombotic complications are not unreported. da Costa et al. reported five cases with nonneurological thrombotic complications in patients with dengue fever (DF).1 We were able to find two case reports of CVT in dengue in literature.2,3 In both these reports, patients improved subsequently with adequate fluids and anticoagulation, with none of them requiring surgical intervention. Our patient had normal hematocrit values and she was being adequately hydrated. So, it looks unlikely that alone dehydration was the cause of her prothrombotic state. Her thrombocheck panel was normal, and she did not carry any prothrombotic risk factors like OCP use or smoking. Several mechanisms have been described in literature for the association between DF and thrombotic processes.4,5
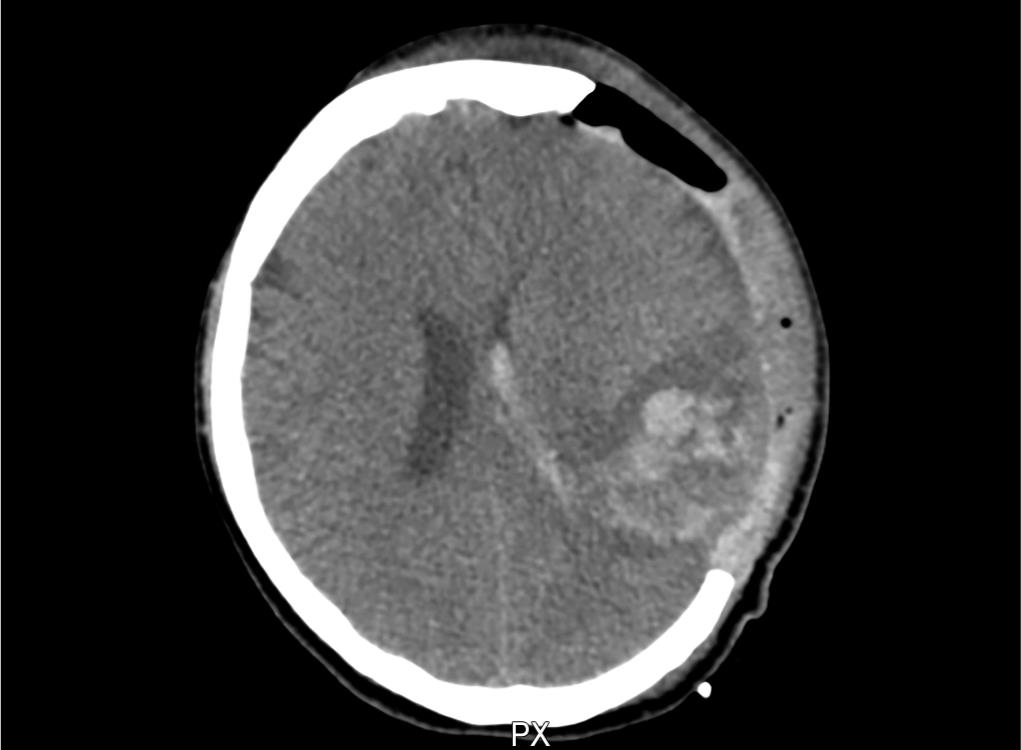
Fig. 1: CT brain showing postoperative status with acute left temporal hematoma
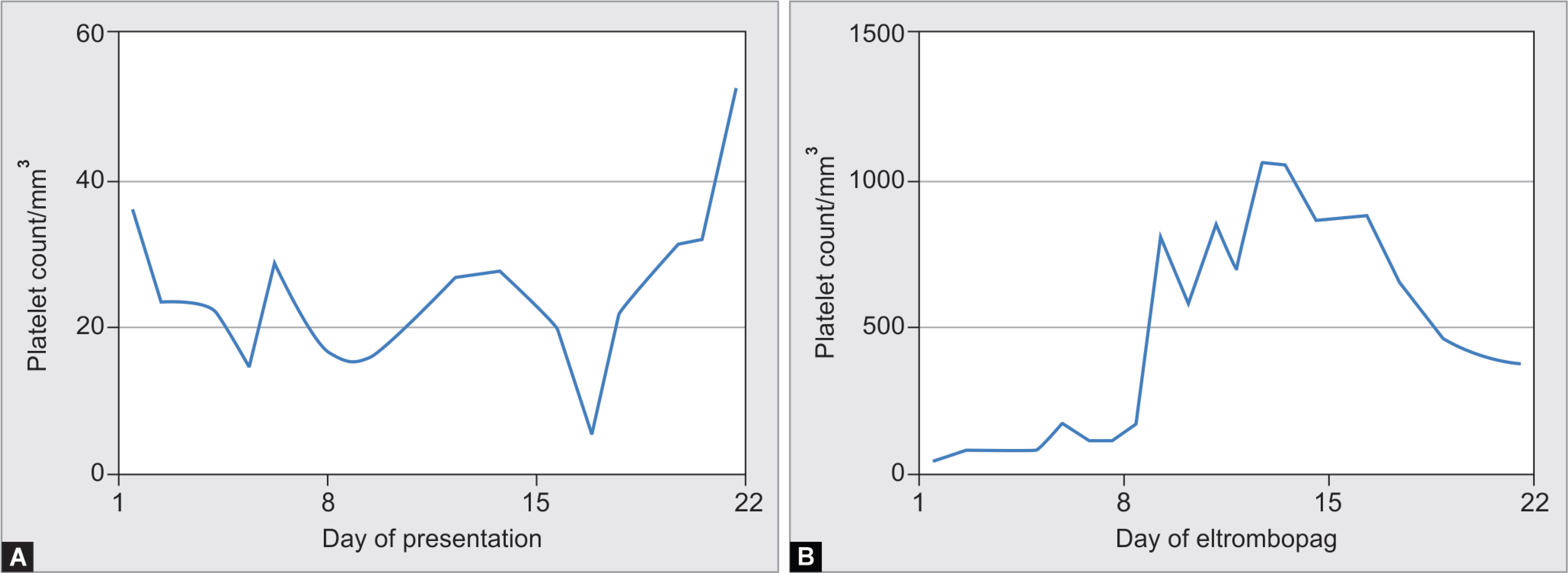
Fig. 2: Figs 2A and B: Platelet count trends before and after eltrombopag
Thrombocytopenia is one of the hallmarks of DF. It usually starts on day 3rd of fever while starts improving beyond 8th–10th day.6–8 The mechanisms involved in thrombocytopenia in dengue are not fully understood and several hypotheses have been suggested to elucidate it.4,9–11 Our patient continued to have low platelet counts in the 3rd week of her illness which was refractory to any transfusion. We treated her as immune thrombocytopenic purpura (ITP) after ruling out other possible causes for thrombocytopenia. There have been case reports of secondary ITP in DF.12,13 High dose dexamethasone 40 mg/day although effective in ITP, has not shown the same results DF.14,15 There are reports in the literature showing success with IVIg for increasing the platelet count in DF.16 Our patient however, remained refractory to these therapies. Lack of response to IVIg was similar to observation by Dimaano et al.17 Eltrombopag is a nonpeptide thrombopoietin receptor (TPO-R) agonist.18 It has been shown to effectively increase platelet counts with chronic ITP with an overall response rate of 60–80%. Platelet counts start to increase after the first week of therapy and peak in the second week.19 Looking at the trends of platelet counts (increase after the first week of therapy followed by thrombocytosis and stabilization after withdrawing the drug), it is quite obvious that our patient responded to eltrombopag (Fig. 2).
It can thus be considered as a potential therapy for life-threatening thrombocytopenia in DF however, further research is needed to prove its efficacy owing to the heterogeneity of mechanisms involved in thrombocytopenia in DF.
REFERENCES
1. da Costa PSG, Ribeiro GM, Soares Junior C, da Costa Campos L. Severe thrombotic events associated with dengue fever, Brazil. Am J Trop Med Hyg 2012;87(4):741–742. DOI: 10.4269/ajtmh.2012.11-0692.
2. Vasanthi N. Unusual presentation of dengue fever-cerebral venous thrombosis. J Clin Diagn Res 2015;9(6):OD09–OD10. DOI: 10.7860/JCDR/2015/13132.6068.
3. Tilara M, Shah AN, Negi S, Dadhania J. A case of cerebral venous thrombosis in the patient with dengue. Int J Sci Res Pub 2014;4(8):1–11.
4. Lin C, Wan S, Cheng H, Lei H, Lin Y. Autoimmune pathogenesis in dengue virus infection. Viral Immunol 2006;19(2):127–132. DOI: 10.1089/vim.2006.19.127.
5. Wills B, Oragui E, Stephens A, Daramola O, Dung N, Loan H, et al. Coagulation abnormalities in dengue hemorrhagic fever: serial investigations in 167 vietnamese children with dengue shock syndrome. Clin Infect Dis 2002;35(3):277–285. DOI: 10.1086/341410.
6. Mitrakul C. Bleeding problem in dengue haemorrhagic fever: platelets and coagulation changes. Southeast Asian J Trop Med Public Health 1987;18(3):407–412.
7. Srichaikul T, Nimmannitya S. Haematology in dengue and dengue haemorrhagic fever. Baillieres Best Pract Res Clin Haematol 2000;13(2):261–276. DOI: 10.1053/beha.2000.0073.
8. Azin FRFG, Goncalves RP, Pitombeira MHDS, Lima DM, Branco IC. Dengue: profile of haematological and biochemical dynamics. Rev Bras Hematol Hemoter 2012;34(1):36–41. DOI: 10.5581/1516-8484.20120012.
9. Nakao S, Lai CJ, Young NS. Dengue virus, a flavivirus, propagates in human bone marrow progenitors and hematopoietic cell lines. Blood 1989;74(4):1235–1240. DOI: 10.1182/blood.V74.4.1235.bloodjournal7441235.
10. Hottz E, Oliveira M, Nunes P, Nogueira R, Valls-de-Souza R, Da Poian A, et al. Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC-SIGN and caspases. J Thromb Haemost 2013;11(5):951–962. DOI: 10.1111/jth.12178.
11. Funahara Y, Sumarmo, Wirawan R. Features of DIC in dengue hemorrhagic fever. Bibl Haematol 1983;49:201–211. DOI: 10.1159/000408460.
12. Ron-Guerrero CS, Barrera-Chairez E, Ron-Magaña AL, Razón-Gutiérrez JE. Trombocitopenia persistente parecida a púrpura trombocitopénica inmune asociada al dengue hemorrágico: informe de tres casos. Rev Hematol Mex 2013;14(2):86–90.
13. Ramírez-Fonseca T, Segarra-Torres A, Jaume-Anselmi F, Ramírez-Rivera J. Dengue fever: a rare cause of immune thrombocytopenia. Bol Asoc Med P R 2015;107(2):51–53.
14. Shashidhara K. Effect of high dose of steroid on plateletcount in acute stage of dengue fever with thrombocytopenia. J Clin Diagn Res 2013;7(7):1397–1400. DOI: 10.7860/JCDR/2013/6135.3143.
15. Kularatne S, Walathara C, Mahindawansa S, Wijesinghe S, Pathirage M, Kumarasiri P, et al. Efficacy of low dose dexamethasone in severe thrombocytopenia caused by dengue fever: a placebo controlled study. Postgrad Med J 2009;85(1008):525–529. DOI: 10.1136/pgmj.2008.078444.
16. Kumar P. Intravenous immunoglobulin responsive persistent thrombocytopenia after dengue haemorrhagic fever. J Clin Diagn Res 2016;10(4):OD10–OD11. DOI: 10.7860/JCDR/2016/17770.7605.
17. Dimaano EM, Saito M, Honda S, Miranda EA, Alonzo MTG, Valerio MD, et al. Lack of efficacy of high-dose intravenous immunoglobulin treatment of severe thrombocytopenia in patients with secondary dengue virus infection. Am J Trop Med Hyg 2007;77(6):1135–1138. DOI: 10.4269/ajtmh.2007.77.1135.
18. Erickson-Miller C, DeLorme E, Tian S, Hopson C, Stark K, Giampa L, et al. Discovery and characterization of a selective, nonpeptidyl thrombopoietin receptor agonist. Exp Hematol 2005;33(1):85–93. DOI: 10.1016/j.exphem.2004.09.006.
19. Cheng G. Eltrombopag, a thrombopoietin-receptor agonist in the treatment of adult chronic immune thrombocytopenia: a review of the efficacy and safety profile. Ther Adv Hematol 2012;3(3):155–164. DOI: 10.1177/2040620712442525.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.