INVITED ARTICLE | https://doi.org/10.5005/jp-journals-10071-23381 |
Furosemide Stress Test in Predicting Acute Kidney Injury Outcomes
1Department of Nephrology, Apollo Hospitals, Chennai, Tamil Nadu, India
2Department of Critical Care Medicine, Apollo Hospitals, Chennai, Tamil Nadu, India
Corresponding Author: Ramesh Venkataraman, Department of Critical Care Medicine, Apollo Hospitals, Chennai, Tamil Nadu, India, Phone: +91 44 28296517, e-mail: ccmramesh@gmail.com
How to cite this article Rajasekaran KK, Venkataraman R. Furosemide Stress Test in Predicting Acute Kidney Injury Outcomes. Indian J Crit Care Med 2020;24(Suppl 3):S100–S101.
Source of support: Nil
Conflict of interest: None
Keywords: Acute kidney injury, Critical care, Furosemide.
INTRODUCTION
Acute kidney injury (AKI) is the most common problem encountered in critical care patients which is associated with significant morbidity and mortality.1,2 Incidence of AKI has doubled in the past decade and is projected to increase in near future.3 Management of AKI in the critical care setting is challenging and involves a wide spectrum of treatment options ranging from conservative supportive care to renal replacement therapy (RRT). Once AKI occurs, the course and consequences vary. There are no clear markers that can be used to predict the progression of AKI or the need for RRT. Moreover, when AKI progresses, there is no clear consensus on the timing of initiation of RRT.4 Multiple biomarkers have been evaluated to predict onset and worsening of AKI and the need for RRT. Biomarkers indicate some structural damage, and elevated biomarkers even in the absence of elevation in serum creatinine have been shown to indicate more severe disease. However, most of these biomarkers are not routinely available for clinical use, and their utility in clinical practice is unclear. The levels of biomarkers also change with severity and timing of injury which make their interpretation difficult.5 Intravenous diuretic administration is often employed to augment urine output in oliguric patients with AKI prior to initiation of RRT. Recently, clinicians have used the response to furosemide to stratify AKI and predict its progression and need of RRT.
FUROSEMIDE STRESS TEST (FST)—RATIONALE
Furosemide, a loop diuretic, is not effectively filtered by the glomerulus, and hence, the tubular concentration of furosemide does not depend on the glomerular filtration rate. Furosemide is transported to the proximal tubule via the peritubular capillaries and then gains access to tubular lumen by active secretion via human organic anionic transporter system in the proximal convoluted tubule. Furosemide then reaches the thick ascending loop of Henle where it inhibits luminal chloride transport decreasing sodium reabsorption leading to natriuresis and increased urine flow.6 Hence, the presence of brisk diuretic response to furosemide indicates reasonably intact renal blood flow, proximal tubular secretory capacity, and function of thick ascending loop of Henle and indicates good functional reserve of the kidneys in patients with AKI. Hence, the increase in urine output after administration of furosemide can be used to assess the integrity of tubular function in patients with early AKI.
FST—EVIDENCE
In 2013, Chawla et al.7 in a single-center study evaluated the utility of diuretic response to a standardized dose of furosemide and attempted to predict the progression of AKI and need for RRT based on the presence or absence of diuresis. Patients with stage I or II AKI [Acute Kidney Injury Network (AKIN) criteria] were administered a standardized dose of furosemide. Patients who were loop-diuretic naive received 1.0 mg/kg of furosemide, while patients previously exposed to furosemide within the previous 7 days received 1.5 mg/kg of furosemide as an intravenous bolus. Post furosemide administration, urine output was measured hourly for 6 hours and in total for 24 hours (Flowchart 1). The treating team could choose to replace the first 6 hours of urine output after furosemide administration with either equal volumes of Ringer’s lactate or normal saline to prevent hypovolemia. Primary outcome evaluated was progression to AKIN stage III within 14 days of FST. Out of 77 patients studied, those with progressive AKI had a significantly lower urine output following FST in each of the first 6 hours (p < 0.001). The area under the receiver operating characteristic curve (AuROC) for the total urine output over the first 2 hours following FST to predict progression to AKIN-III was 0.87 (p = 0.001). The authors determined that the ideal cutoff for predicting AKI progression during the first 2 hours following FST was a urine volume of <200 mL (100 mL/hour) with a sensitivity of 87.1% and a specificity of 84.1%. In other words, patients who did not have urine output of 200 mL within 2 hours after furosemide administration were more likely to progress to AKIN-III.
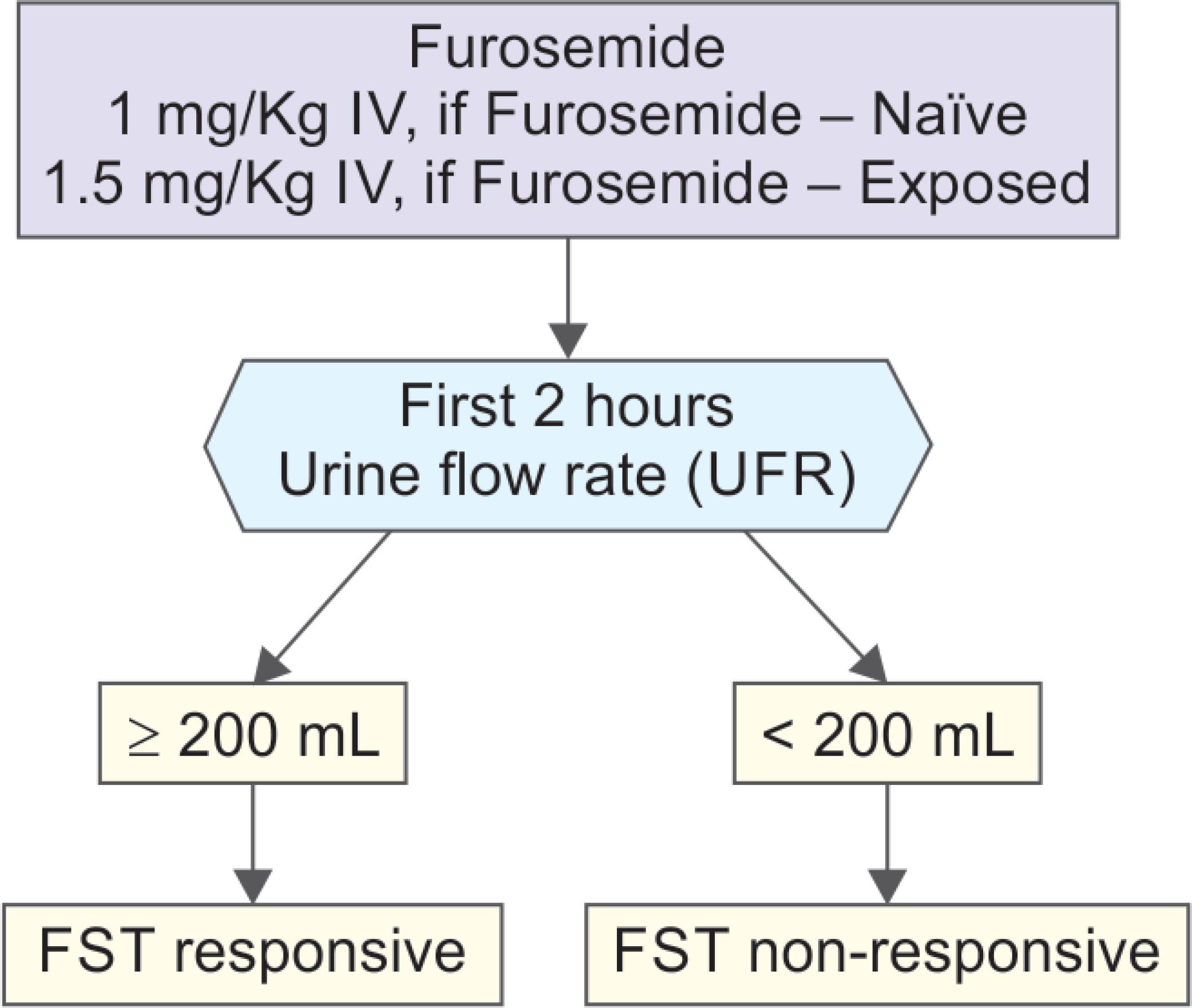
Flowchart 1: Furosemide stress test
Subsequently, Koyner et al. compared the performance of FST to a multitude of biomarkers for predicting the severity of AKI.8 They demonstrated that biomarkers did not perform significantly better than the FST for predicting progression to stage III AKI, the need for RRT, or mortality. However, when FST was combined with the other biomarkers of AKI, there was an improvement in risk prediction for all outcomes.
More recently, Rewa et al.9 performed a multicenter, prospective, observational study in patients with stage I or II AKI. After performing FST, the investigators observed that urine flow rate during the first 2 hours was the most predictive of progression to stage III AKI (AuROC = 0.87), with an ideal cutoff of less than 200 mL, with a sensitivity of 73.9% and a specificity of 90.0%. In another multicenter study, patients were administered FST and FST nonresponders were then randomized to either standard or early RRT.10 In the early group, RRT was carried out within 6 hours of randomization. In the standard group, RRT was initiated only if prespecified indications were met. The study demonstrated that among FST responsive patients, only 13.6% ended up needing RRT. Among 118 patients who did not respond to FST, 98.3% in the early RRT group and 75% in the standard RRT group needed RRT. Furosemide stress test seems to be a good strategy to identify patients who may benefit from early RRT.
CONCLUSION
Considering the wide spectrum of factors contributing to AKI in the intensive care unit and its impact on morbidity and mortality, strategies to help understand its course early and guide therapy accordingly are lacking for most part. Some of the newer biomarkers although promising and validated in large studies are not widely available for clinical use and may be cost-ineffective when done frequently. Furosemide stress test seems to be a simple bedside tool that performs reasonably well in identifying patients at high risk of AKI progression and need for RRT. Moreover, the utility of biomarkers may be enhanced when combined with FST response. Further studies exploring the utility of FST in AKI stratification and as a tool to target interventions and RRT in high-risk patients are warranted.
REFERENCES
1. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 2004;8(4):R204–R212. DOI: 10.1186/cc2872.
2. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012;81(5):442–448. DOI: 10.1038/ki.2011.379.
3. Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol 2013;24(1):37–42. DOI: 10.1681/ASN.2012080800.
4. Gibney N, Hoste E, Burdmann EA, Bunchman T, Kher V, Viswanathan R, et al. Timing of initiation and discontinuation of renal replacement therapy in AKI: unanswered key questions. Clin J Am Soc Nephrol 2008;3(3):876–880. DOI: 10.2215/CJN.04871107.
5. Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol 2007;156:203–212. DOI: 10.1159/000102085.
6. Hasannejad H, Takeda M, Taki K, Shin HJ, Babu E, Jutabha P, et al. Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther 2004;308(3):1021–1029. DOI: 10.1124/jpet.103.059139.
7. Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care 2013;17(5):R207. DOI: 10.1186/cc13015.
8. Koyner JL, Davison DL, Brasha-Mitchell E, Chalikonda DM, Arthur JM, Shaw AD, et al. Furosemide stress test and biomarkers for the prediction of AKI severity. J Am Soc Nephrol 2015;26(8):2023–2031. DOI: 10.1681/ASN.2014060535.
9. Rewa OG, Bagshaw SM, Wang X, Wald R, Smith O, Shapiro J, et al. The furosemide stress test for prediction of worsening acute kidney injury in critically ill patients: a multicenter, prospective, observational study. J Crit Care 2019;52:109–114. DOI: 10.1016/j.jcrc.2019.04.011.
10. Limlertgul N, Peerapornratana S, Trakarnvanich T, Pongsittisak W, Surasit K, Chuasuwan A, et al. Early versus standard initiation of renal replacement therapy in furosemide stress test non-responsive acute kidney injury patients (the FST trial). Crit Care 2018;22(1):101. DOI: 10.1186/s13054-018-2021-1.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.