CASE REPORT | https://doi.org/10.5005/jp-journals-10071-23586 |
Hypocalcemic Seizure Due to Vitamin D Deficiency
1,6,7Department of Internal Medicine, Baby Memorial Hospital, Kozhikode, Kerala, India
2Department of Cardiology, Baby Memorial Hospital, Kozhikode, Kerala, India
3Department of Endocrine Surgery, Baby Memorial Hospital, Kozhikode, Kerala, India
4Department of Cardiothoracic and Vascular Surgery, Baby Memorial Hospital, Kozhikode, Kerala, India
5Department of Neurology, Baby Memorial Hospital, Kozhikode, Kerala, India
Corresponding Author: Robin G Manappallil, Department of Internal Medicine, Baby Memorial Hospital, Kozhikode, Kerala, India, Phone: +91 8547753396, e-mail: drrobingeorgempl@gmail.com
How to cite this article Manappallil RG, Krishnan R, Veetil PP, Nambiar H, Karadan U, Anil R, et al. Hypocalcemic Seizure Due to Vitamin D Deficiency. Indian J Crit Care Med 2020;24(9):882–884.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Aim: To emphasize the importance of vitamin D supplementation.
Background: The incidence of vitamin D deficiency has been increasing worldwide, probably due to decreased exposure to sunlight and unbalanced diet. Severe hypocalcemia following vitamin D deficiency is rather uncommon, and this leading to seizures in adults is a rare scenario.
Case description: This is the case of a 70-year-old female, a known case of coronary artery disease, who presented with one episode of seizure. Computed tomography of her brain revealed diffuse age-related atrophic changes, and electroencephalogram showed diffuse cerebral dysfunction. She was found to have severe hypocalcemia with secondary hyperparathyroidism due to vitamin D deficiency. Vitamin D bolus was given along with calcium correction, following which she improved.
Conclusion: There are a few reports of hypocalcemic seizures among children; however, the incidence is rare among adults. Calcium and vitamin D supplementation forms the mainstay of treatment.
Clinical significance: Hypocalcemic seizure is uncommon, especially among adults. Vitamin D deficiency resulting in hypocalcemic seizure, to the best of our knowledge, is an unreported scenario. This case highlights the importance of vitamin D supplementation in those with reduced sunlight exposure.
Keywords: Calcium, Hyperparathyroidism, Hypocalcemia, Seizure, Vitamin D.
INTRODUCTION
Hypocalcemia is a frequently encountered biochemical abnormality. Its severity can range from asymptomatic cases to life-threatening situation. Vitamin D deficiency is a common cause, but severe hypocalcemia is usually associated with iatrogenic hypoparathyroidism in a post-thyroidectomy patient.1–3 Other causes include autoimmune hypoparathyroidism, metastatic or heavy metal infiltration of parathyroid gland, end-stage renal or liver disease, hypo or hypermagnesemia, Hungry bone syndrome, and Fanconi syndrome.4 Some of the drugs responsible for hypocalcemia are bisphosphonates, antiepileptics, aminoglycosides, cisplatin, diuretics, and proton pump inhibitors.5 The symptoms of hypocalcemia include muscle spasms or cramps, paresthesia, tetany, laryngospasm, cognitive impairment, neuromuscular irritability, personality disturbances, seizures, prolonged QT intervals, and heart failure.1,2,6 Serum calcium levels are regulated by parathyroid hormone (PTH), vitamin D, and calcitonin through their effects on the bowel, kidneys, and skeleton.1 The biologically active form of vitamin D, 1,25-dihydroxyvitamin D (1,25[OH]2 D), enhances the intestinal absorption of calcium and phosphorus. Hence, vitamin D deficiency leads to about 50% reduction in gastrointestinal absorption of calcium.7
The patient being described is an elderly female with coronary artery disease who presented with hypocalcemic seizures due to vitamin D deficiency.
CASE DESCRIPTION
A 70-year-old female was brought with history of one episode of generalized tonic–clonic seizure lasting for less than 1 minute. The episode was associated with frothing from mouth, with no tongue bite or bowel and bladder incontinence. She had a post-ictal phase lasting for about 15 minutes. There were no seizure episodes in the past. She also complained of frequent muscle cramps for the past 3 months. She was diagnosed to have coronary artery disease (triple-vessel disease) about 1 month ago and advised coronary artery bypass graft (CABG). Her oral medication list comprised of aspirin (75 mg once daily), clopidogrel (75 mg once daily), atorvastatin (40 mg once daily), glyceryl trinitrate sustained release (2.6 mg twice daily), torsemide (5 mg once daily), and metoprolol (25 mg twice daily). She was mainly confined to her house, and her diet was devoid of milk and milk products for many years.
On presentation, she was conscious and oriented. She was afebrile, with pulse rate of 80/minutes, blood pressure 130/80 mm Hg, and respiratory rate 20/minutes (with saturation 94% rom air). Her systemic examinations were normal, with no signs of meningeal irritation. Trousseau sign was positive but Chvostek sign was negative.
Her blood investigations such as complete blood counts, renal and liver functions, electrolytes, random blood glucose, HbA1c, TSH, and plasma ammonia were normal. Serum calcium was 6.4 mg/dL, serum albumin was 2.9 mg/dL, and corrected calcium was 6.9 mg/dL (8.7–10.2). Serum magnesium and phosphorus were normal. Vitamin D3 level was 7 ng/mL (30–100), and PTH levels were 324 ng/L (8–51). Computed tomography of the brain showed diffuse age-related atrophic changes, electroencephalogram was suggestive of diffuse cerebral dysfunction (high amplitude activity with slow waves in the posterior head regions which were bilaterally responsive to eye opening; no epileptiform activities were seen), and cerebrospinal fluid analysis was normal. Electrocardiography showed mild ST-segment depression in anterolateral leads with T-wave inversion in V1-4, T-wave flattening in V5-6 with QT prolonged (Fig. 1), and echocardiography had regional wall motion abnormality, mild mitral regurgitation, and moderate left ventricular systolic and diastolic dysfunction with 35% ejection fraction. Ultrasound of her neck did not show any thyroid or parathyroid abnormality. Ultrasound of abdomen and chest X-ray were normal. Her 24-hour urinary calcium was normal (100 mg).
She was given single bolus dose of intramuscular cholecalciferol (3 lakh IU) and intravenous 10% calcium gluconate (20 mL), followed by 10% calcium gluconate infusion (1.5 mg/kg/hour). Oral sodium valproate (300 mg twice daily) was started in view of possibility of recurrence of seizure. Her serial calcium levels started showing an increasing trend and got normalized (9.3 mg/dL) by day 4 of admission. Calcium infusion was stopped and changed to combination tablet of calcium carbonate (500 mg elemental calcium) with vitamin D3 (250 IU) thrice daily, along with oral calcitriol (0.25 μg twice daily). Following normalization of calcium levels, she was taken up for CABG. Her postoperative period was uneventful and was discharged on day 10 of admission on oral calcium with vitamin D3 (500 mg/250 IU once daily), calcitriol (0.25 mg once daily), sodium valproate (300 mg twice daily), aspirin (75 mg once daily), clopidogrel (75 mg once daily), atorvastatin (40 mg once daily), glyceryl trinitrate sustained release (6.4 mg twice daily), torsemide (10 mg once daily), and metoprolol (25 mg twice daily). On review after 2 weeks, her calcium levels were normal. Calcitriol was stopped, and oral calcium with vitamin D3 (500 mg/250 IU once daily) was continued. Her calcium, parathyroid hormone, and vitamin D3 levels after 1 month of CABG were 9.2 mg/dL, 34 ng/L, and 47 ng/mL, respectively. She did not have any further episodes of seizure. Sodium valproate was tapered and stopped, with continuation of oral calcium with vitamin D3 (500 mg/250 IU once daily) along with routine cardiac medications. She was advised milk and milk products in her diet and regular monthly monitoring of calcium and vitamin D3 levels. The course of her calcium levels is depicted in Figure 2.
DISCUSSION
Calcium homeostasis is maintained by the coordinated actions of PTH and vitamin D. PTH stimulates calcium resorption from the renal tubules and release of calcium from the bones. PTH also stimulates the renal production of 1,25(OH)2D from 25-hydroxycholecalciferol (25[OH]D). 1,25(OH)2D is the active form of vitamin D and promotes the absorption of calcium from the small intestine.
Vitamin D deficiency is the most common cause of hypocalcemia and is due to inadequate exposure to sunlight, skin pigmentation, skin thinning with age, insufficient dietary intake, antiepileptic drugs, and malabsorption.1,7–9 Supplementation of vitamin D is essential to prevent rickets and osteoporosis. Calcium and vitamin D supplements have also helped to prevent hip fractures among geriatric population. Vitamin D aids in decreasing the incidence of cardiovascular diseases, cancer, inflammatory, and autoimmune diseases.7,10,11
The Institute of Medicine 2010 report has recommended daily vitamin D dose of 600 IU for those below 70 years and 800 IU for above 70 years of age. The National Institute of Health recommends daily calcium intake of 1,000–1,200 mg in adults. Vitamin D deficiency states are managed with weekly dosing of 50,000 IU for 3–12 weeks, followed by maintenance doses of 800 IU daily along with calcium.12 Large single doses of 250,000–300,000 IU of vitamin D have shown to be effective at improving vitamin D levels and suppressing PTH concentrations.13,14
With only a handful of cases being reported, hypocalcemic seizures are uncommon and are seen mainly in infants and children.15,16 Our patient was an elderly lady who presented with hypocalcemic seizure due to vitamin D deficiency. To the best of our knowledge, such a scenario has not been reported yet.
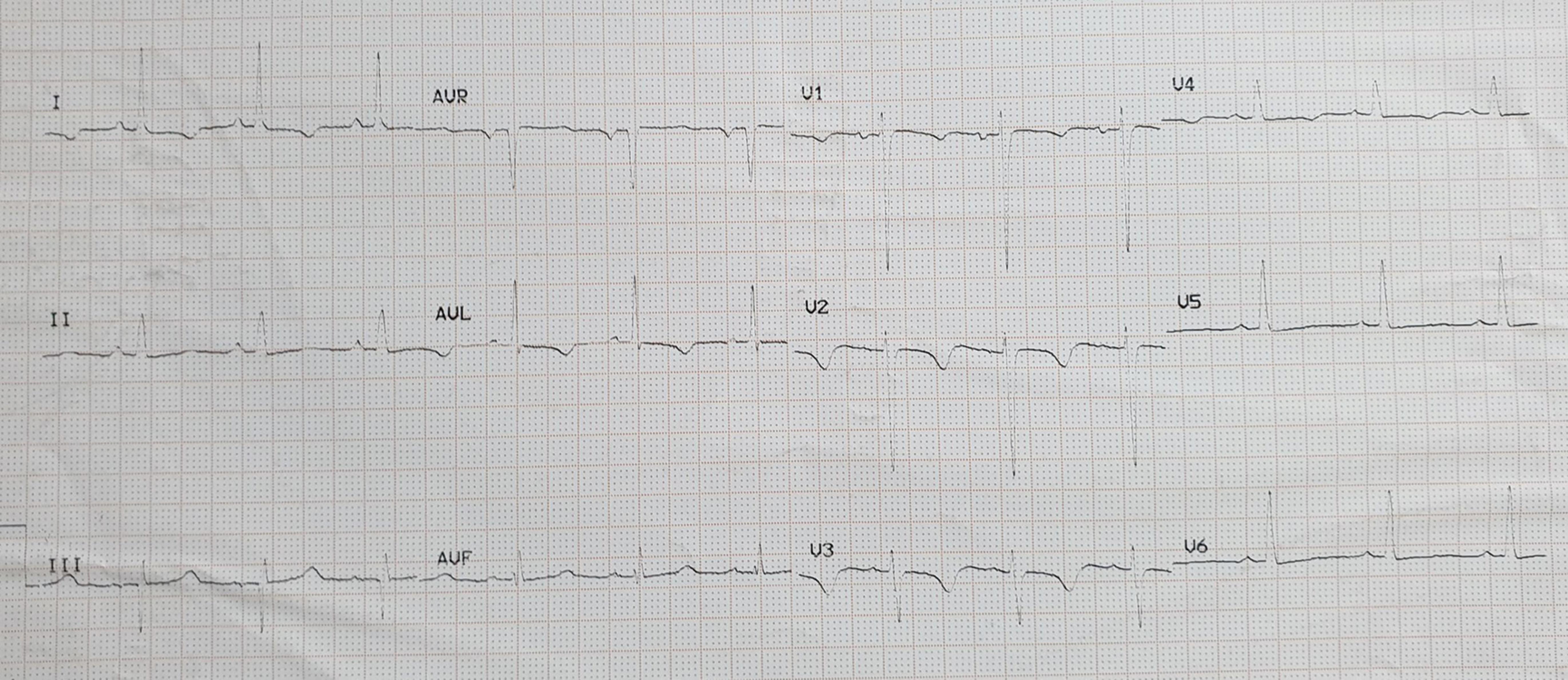
Fig. 1: Electrocardiogram showing mild ST segment depression in anterolateral leads with T wave inversion in V1-4, T wave flattening in V5-6 with QT prolonged
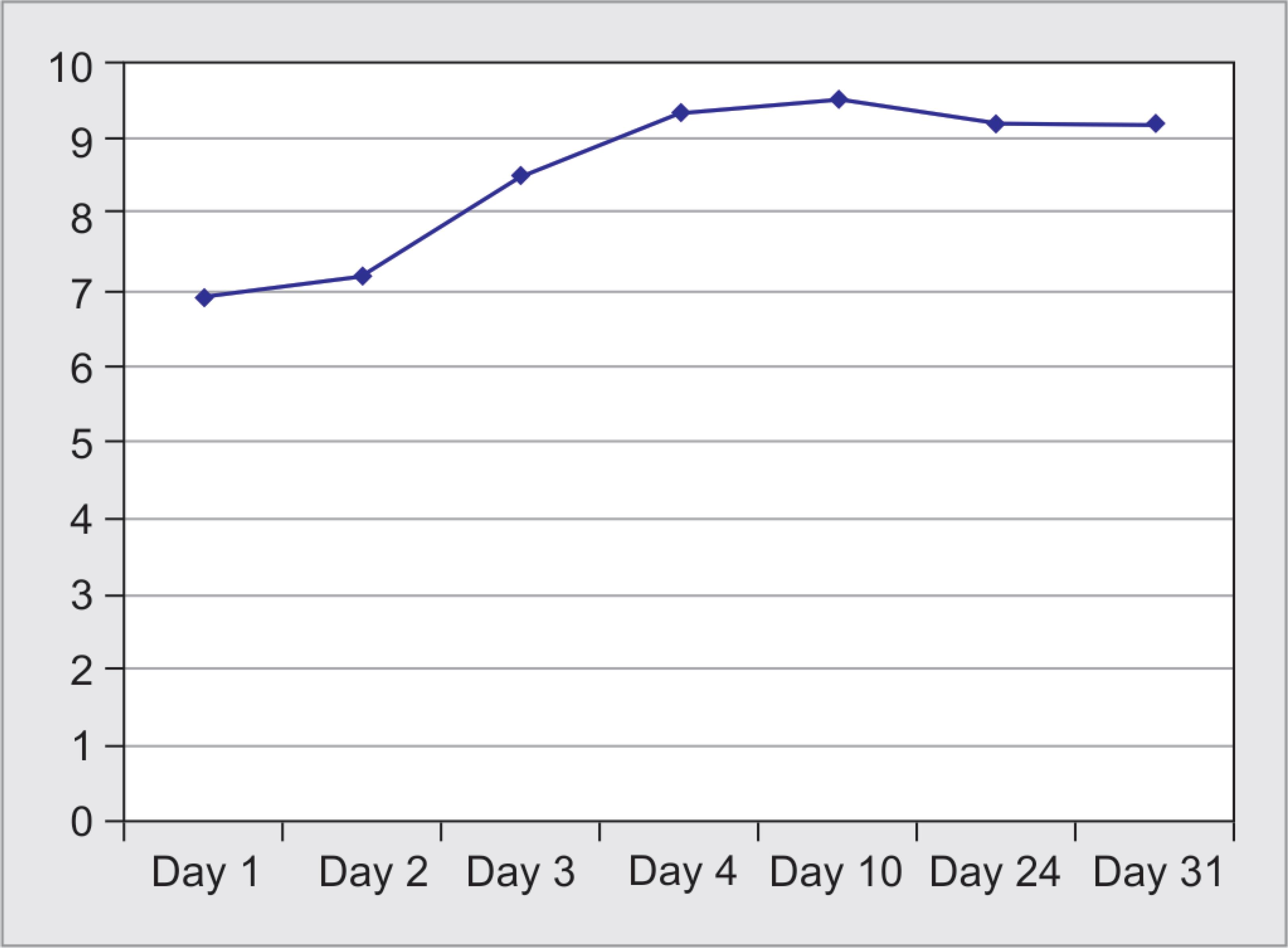
Fig. 2: Course of calcium levels
CONCLUSION
Vitamin D is required for the intestinal absorption of calcium. Sunlight is the prime source of vitamin D. However, in our current lifestyle, exposure to sunlight is less and food alone might not be sufficient, thereby leading to hypocalcemia. Hence, dietary vitamin D supplementation in the form of daily or once weekly vitamin D preparations is essential. In the current case, the patient had severe vitamin D deficiency, leading to hypocalcemia and presented as seizure.
AUTHORS’ CONTRIBUTIONS
Robin G Manappallil: Concept and design, manuscript preparation, review of literature and treating Physician; Raghuram Krishnan: Critical revision of manuscript and treating Cardiologist; Pradeep P Veetil: Critical revision of manuscript and treating Endocrine Surgeon; Ummer Karadan: Critical revision of manuscript and treating Neurologist; Harilal Nambiar: Critical revision of manuscript and treating Cardiothoracic Surgeon; Revathy Anil: Resident in-charge; Blessy Josephine: Resident in-charge
REFERENCES
1. Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008;336(7656):1298–1302. DOI: 10.1136/bmj.39582.589433.BE.
2. Murphy E, Williams GR. Hypocalcaemia. Medicine 2009;37(9):465–468. DOI: 10.1016/j.mpmed.2009.06.003.
3. Prendiville S, Burman KD, Wartofsky L, Ringel M, Sessions R. Evaluation and treatment of post-thyroidectomy hypocalcemia. Endocrinologist 1998;8(1):34. DOI: 10.1097/00019616-199801000-00008.
4. Fong J, Khan A. Hypocalcemia: updates in diagnosis and management for primary care. Can Fam Physician 2012;58(2):158–162.
5. Liamis G, Milionis HJ, Elisaf M. A review of drug-induced hypocalcemia. J Bone Miner Metab 2009;27(6):635–642. DOI: 10.1007/s00774-009-0119-x.
6. Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med 2008;359(4):391–403. DOI: 10.1056/NEJMcp0803050.
7. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357(3):266–281. DOI: 10.1056/NEJMra070553.
8. Gloth FM, Gundberg CM, Hollis WB, Haddad JG, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA 1995;274(21):1683–1686. DOI: 10.1001/jama.1995.03530210037027.
9. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol 2011;96(7):1911–1930. DOI: 10.1210/jc.2011-0385.
10. Jacobsen RB, Hronek BW, Schmidt GA, Schilling ML. Hypervitaminosis D associated with a vitamin D dispensing error. Ann Pharmacother 2011;45(10):e52. DOI: 10.1345/aph.1Q330.
11. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 2010;10(4):482–496. DOI: 10.1016/j.coph.2010.04.001.
12. Bringhurst FR, Demay MB, Krane SM, Kronenberg HM. Bone and mineral metabolism in healthy and disease ed. F, Kasper L, Hauser L, Jameson ed. Harrison’s principles of internal medicine.New York, USA: McGraw Hill Education; 2015. pp. 2463–2466.
13. Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract 2014;20(4):341–351. DOI: 10.4158/EP13265.RA.
14. Kearns MD, Binongo JN, Watson D, Alvarez JA, Lodin D, Ziegler TR, et al. The effect of a single, large bolus of vitamin D in healthy adults over the winter and following year: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr 2015;69(2):193–197. DOI: 10.1038/ejcn.2014.209.
15. Thirunavukarasu S, Devi V, Usheva S, Arumugam D, Ramanujam A, et al. A rare case of acute symptomatic seizure, tetany and poor sensorium secondary to autosomal dominant hypocalcemia and hypercalciuria. J Rare Disord Diagn Ther 2018;4:2.
16. Kossoff EH, Silvia MT, Maret A, Carakushansky M, Vining EP. Neonatal hypocalcemic seizures: case report and literature review. J Child Neurol 2002;17(3):236–239. DOI: 10.1177/088307380201700319.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.