ORIGINAL RESEARCH |
https://doi.org/10.5005/jp-journals-10071-23790 |
Microbiology of Ventilator-associated Pneumonia in a Tertiary Care Cancer Hospital
1Department of Microbiology, Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai, Maharashtra, India
2Department of Microbiology, Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Mumbai, Maharashtra, India
3,4Department of Microbiology, Tata Memorial Centre, Mumbai, Maharashtra, India
Corresponding Author: Vivek Bhat, Department of Microbiology, Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Mumbai, Maharashtra, India, Phone: +91 9867793325, e-mail: vivekbhat2005@yahoo.com
How to cite this article: Sangale A, Bhat V, Kelkar R, Biswas S. Microbiology of Ventilator-associated Pneumonia in a Tertiary Care Cancer Hospital. Indian J Crit Care Med 2021;25(4):421–428.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Background: Ventilator-associated pneumonia (VAP) is an important cause of healthcare-associated infections, resulting in prolonged hospitalization with increased morbidity and mortality. Knowledge of predominant local pathogens and their antimicrobial susceptibility patterns helps in selection of appropriate initial antibiotic therapy in these critical cases.
Aim and objective: The aim and objective of this study is to characterize the microbiology and antimicrobial susceptibility patterns of VAP isolates in a tertiary cancer center.
Materials and methods: This is a 4-year qualitative observational study carried out at a tertiary care cancer hospital in Mumbai. All nondirect bronchoalveolar lavage specimens from patients with a clinical suspicion of VAP sent from the critical care unit to the department of microbiology were processed as per standard laboratory procedures. All isolates were identified to species level and an antimicrobial susceptibility testing was performed by the Kirby–Bauer disk diffusion method and/or the VITEK 2 automated identification and susceptibility system, according to Clinical and Laboratory Standards Institute guidelines.
Results: The study comprised 1,074 patients: 710 (66.10%) men and 364 (33.90%) women. A total of 827 bacterial isolates were obtained with 780 (94.32%) gram-negative organisms and 47 (5.68%) gram-positive organisms; of which Acinetobacter baumannii (38.7%), Pseudomonas aeruginosa (17.5%), and Klebsiella pneumoniae (16.6%) were the commonest. Of gram-negative bacilli, multidrug-resistant organisms constituted 87.50% and were susceptible to colistin.
Conclusions: VAP is associated with pathogens, such as A. baumannii, P. aeruginosa, and K. pneumoniae in our setting. High rates of resistance to aminoglycosides, β-lactam-β-lactamase inhibitor combinations, and carbapenems were noted.
How to cite this article: Sangale A, Bhat V, Kelkar R, Biswas S. Microbiology of Ventilator-associated Pneumonia in a Tertiary Care Cancer Hospital. Indian J Crit Care Med 2021;25(4):421–428.
Keywords: Carbapenem-resistant A. baumannii (CRAB), Multidrug-resistant organisms, Nondirect bronchoalveolar lavage, Ventilator-associated pneumonia.
INTRODUCTION
The use of mechanical ventilation in patients with respiratory failure has modernized the management of critically ill patients. Ever since its first description in the 1950s, the use of ventilators has increased several folds.1 It has become an essential feature of modern critical care but is associated with complications, such as those during intubation, ventilator-induced lung injury, and the most prominent being ventilator-associated pneumonia (VAP), which leads to prolonged hospitalization, morbidity, and mortality.
According to the American Thoracic Society, VAP is defined as “pneumonia occurring more than 48 hours after the initiation of endotracheal intubation and mechanical ventilation.”2 It is the inflammation of lung parenchyma caused by infectious agents not present or incubating at the time mechanical ventilation was started.3 VAP is a subgroup of healthcare-associated infections and it is a critical device-associated infection (DAI) observed in an intensive care unit (ICU) setting.4 It is one of the leading causes of death contributing to morbidity and mortality in ventilated patients.5
Cancer patients generally get admitted to ICU for multiorgan dysfunction, mainly respiratory failure originating from infectious, malignant, or toxic complications.6 Aggressive antineoplastic chemotherapy makes cancer patients immunocompromised, thus more susceptible to infections. The severity of underlying diseases and exposure to invasive procedures and critical devices result in high mortality and considerable expenditure in these critically ill patients.7
Our hospital is one of the largest tertiary care centers providing critical care facilities to cancer patients in the country. The ICU is divided into postsurgical and medical ICUs and recovery rooms and provides high-quality intensive care for the management of illnesses associated with major surgery and chemotherapy. The most frequently isolated pathogens from patients with VAP are gram-negative bacteria, namely Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli, and gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) reported from hospitals in western as well as Indian literature.8-11
The incidence of VAP has been observed to vary considerably from study to study. In early studies in the 1990s, it was reported to be 16.5% by Papazian et al., in France.12 In later years, Al Dorzi et al., during 2003 to 2008, reported VAP in 14.5% of patients.13 Recent Indian studies conducted by Joseph et al.14 showed the incidence of VAP to be 18%. A similar study conducted by Dey et al. reported an incidence of 45.4%.14 In the western literature, VAP rates varied from 6 to 52%.3,8,16 Indian studies indicate an overall incidence rate of 9 to 58%.10,14,17 It is also observed that surgical ICUs have higher rates of VAP compared to the medical ICUs.9
Mortality rates in patients with VAP are different in general versus cancer hospitals. Papazian et al.,12 conducted a 4-year study on 2,065 general patients in France and found the mortality rate to be 40%. In a multicenter study by Groeger et al., they 6 analyzed 782 adult cancer patients from five tertiary hospitals observing a mortality rate of 76%; of which 41% were from the leukemia group, 20% from the lymphoma group, and 39% were from the solid tumor group. A multicenter, prospective cohort surveillance18 of DAI done in 55 ICUs of 46 hospitals between 2002 and 2005 reported a crude mortality rate of patients without HCAI to be 17.1 and 44.9% with VAP.
A prospective study reported from India by Joseph et al.14 among 200 patients over a period of 15 months in the year 2006 showed a mortality rate of 16.2%. Therefore, the mortality rates of VAP have been reported to range between 0 and 54% according to western as well as Indian data,10,19 while in immunocompromised patients it is 73.3 to 76% as per the western literature.20,21
Intubation compromises the natural barrier between the oropharynx and trachea and helps entry of bacteria into the lung by aspiration and leakage of contaminated secretions around the endotracheal tube cuff.2,9 Studies have shown that upon admission to ICU, in critically ill patients the oral flora shifts to enteric gram-negative bacilli, Acinetobacter spp., P. aeruginosa, and staphylococci. In mechanically ventilated patients, bacterial adherence is favored by denuded mucous membrane, elevated airway pH, and increased numbers of airway receptors for bacteria.22 The stomach has been implicated as a potential reservoir for antibiotic-resistant bacteria, particularly in late-onset VAP.23 Other sources of microorganisms include the paranasal sinuses, dental plaque, and the subglottic area between the true vocal cords and the endotracheal tube cuff.3 A rare mechanism of VAP may result due to macroaspirations of gastric material.9 Efforts should be directed on the prevention of VAP using good infection control practices, hand hygiene, ventilator care bundle approach, and appropriate empirical antibiotic therapy.
The objective of this study is to identify the pathogens associated with the development of VAP and characterize their antimicrobial susceptibility patterns in patients who were put on mechanical ventilation in medical and surgical ICUs of the hospital. This data served as an indicator of microbial trends and susceptibility patterns.
MATERIALS AND METHODS
This was a qualitative observational 4-year study carried out in our tertiary care cancer center in Mumbai and was approved by institutional ethics committee.
The inclusion criteria for the study was nondirect bronchoalveolar lavage (NDBAL) specimens from ventilated patients, showing the presence of at least moderate amounts of polymorphonuclear cells on gram staining, i.e., 10–25 per low power field.24
NDBAL samples from patients on ventilators received in the department of microbiology were processed quantitatively.3 A loopful of specimen was mixed with 1mL normal saline and vortexed for about a minute. Gram stain of smear was examined microscopically for polymorphonuclear cells and the presence of bacteria. Quantitative cultures were performed by a calibrated Nichrome loop of 4 mm internal diameter to pick 0.01 mL volume of the delivered specimen. This was plated on sheep blood agar, MacConkey’s agar (MA), and chocolate agar (CA) using the standard T-streak technique. The blood agar and MA agar plates were incubated aerobically at 35°C. CA was incubated at 35°C in a CO2 incubator with 5% of CO2. The plates were observed for growth at 24 hours and 48 hours.
Duplicate NDBAL specimens from the previously included patients were excluded from the study.
Interpretation of Growth
A colony count of ≥104 colony-forming unit/mL (cfu/mL) was recorded significant25,26 and counts less than this were considered insignificant. Plates that showed significant growth were studied for colony morphology and gram stain. Identification of the organism to species level was done using standard bacteriological methods. For identification of organisms of non-aeruginosa Pseudomonas spp. and those that could not be identified by standard manual biochemicals, VITEK® 2™ compact system was used. This is an automated system for bacterial identification by biochemical analysis using colorimetric technology. The system uses unique identification and antibiotic susceptibility testing cards where the cards and the samples are linked virtually. Antimicrobial susceptibility testing was performed by the Kirby–Bauer disk diffusion method on Mueller–Hinton agar according to Clinical and Laboratory Standards Institute (CLSI) guidelines.25
For susceptibility of antimicrobials like vancomycin, minimum inhibitory concentration (MIC) test was performed using the MIC E-strip method according to CLSI standards.25 Qualitative data were represented in the form of frequency and percentages. SPSS Version 22 was used for the statistical analysis.
RESULTS
A total of 1,608 NDBAL specimens were received by the department of microbiology for over 4 years. Of these, 1,306 NDBAL specimens from 1,074 patients were included in the study as per the inclusion criteria. Of 1,074 cases, 479 showing no bacterial growth or insignificant growth (<104 cfu/mL) on microbiological examination were excluded from the study. There were 595 cases with significant bacterial growth (≥104cfu/mL). Specimens received were from 710 (66.10%) males and 364 (33.90%) females. Of these, 191 (17.8%) patients were in the age-group of <15 years, 110 (10.2%) were in the age-group of 16–30 years, 147 (13.7%) patients in the age-group of 31–45 years, 338 (31.5%) patients in the age-group of 46–60 years, and 288 (26.8%) patients in the age-group of >60 years as shown in Figure 1. The median age was 50 years. The service-wise distribution of cases is shown in Table 1. The highest number of cases were with hematolymphoid malignancies (27.84%) followed by thoracic (25.70%), head and neck malignancies (16.39%), and gastrointestinal and hepatobiliary malignancies (9.78%). Of the 299 hematolymphoid malignancy cases, 133 were diagnosed as acute lymphoblastic leukemia, 65 cases were with acute myelogenous leukemia, 16 with Hodgkin’s lymphoma, two cases with chronic lymphocytic leukemia, and the remaining 83 were acute leukemia (not classified as lymphoblastic or myeloid type).
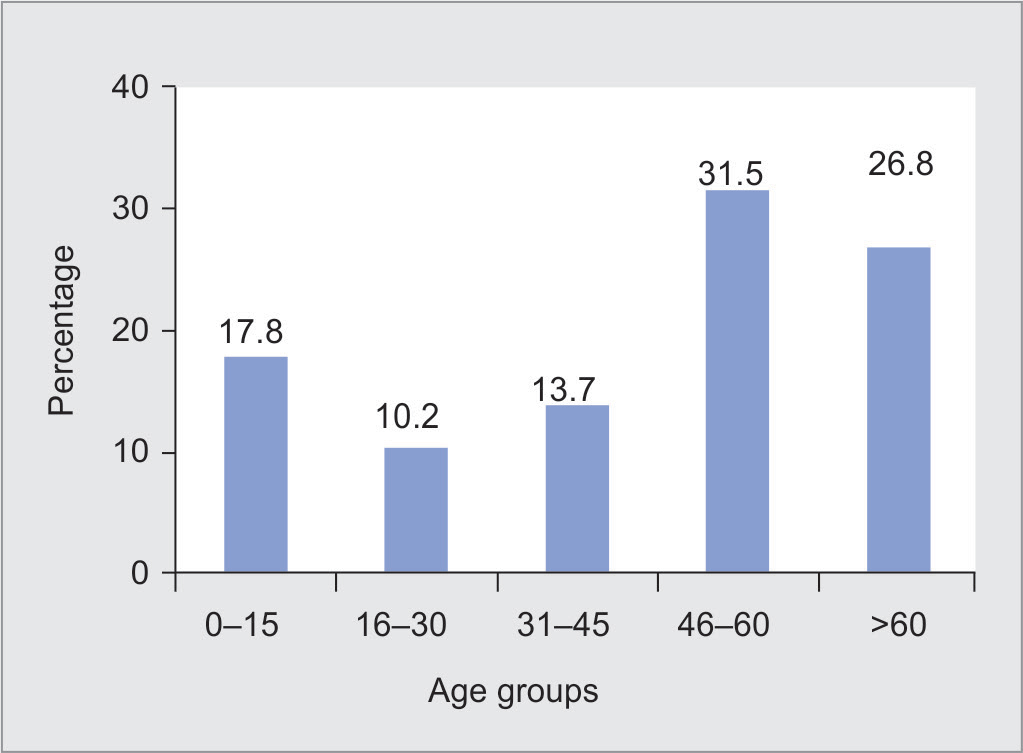
Fig. 1: Age distribution of cases
| Malignancies | No. of patients (n = 1,074) |
Percentage |
|---|---|---|
| Hematolymphoid | 299 | 27.84 |
| Thoracic | 276 | 25.7 |
| Head and neck | 176 | 16.39 |
| Gastrointestinal and hepatobiliary | 105 | 9.78 |
| Bone soft tissue | 41 | 3.82 |
| Genitourinary | 36 | 3.35 |
| Gynecologic | 33 | 3.07 |
| Breast | 12 | 1.12 |
| Central nervous system | 11 | 1.02 |
| Other malignancies | 85 | 7.91 |
| Total | 1,074 | 100 |
The distribution pattern of microbial isolates from NDBAL specimens with significant growth is shown in Table 2. A total of 827 bacterial isolates were obtained from 595 cases. These included 780 (94.32%) gram-negative organisms, of which the commonest organism isolated was A. baumannii (38.7%) followed by P. aeruginosa (17.5%) and K. pneumoniae (16.6%). Among the 47 (5.68%) gram positives, there were 10 isolates of S. aureus, eight isolates of MRSA, 17 Streptococcus pyogenes, five Streptococcus pneumoniae, and two group D streptococci. Further speciation was not available in two cases. Enterococci were isolated in two cases, of which one was vancomycin-resistant Enterococcus (VRE).
Antimicrobial Susceptibility Patterns
Antimicrobial susceptibility pattern of gram-negative isolates is shown in Table 3. Antimicrobial susceptibility pattern of gram-positive isolates was studied and is shown in Table 4.
Of 18 isolates of S. aureus, there were eight MRSA. There was one isolate of VRE. All S. aureus isolates were susceptible to vancomycin, teicoplanin, and linezolid. Nine were resistant to gentamicin and ciprofloxacin and six to erythromycin. All MRSA were resistant to ciprofloxacin and erythromycin and six were resistant to gentamicin and clindamycin. All five isolates of S. pneumoniae were susceptible to penicillin.
| Microbial isolates | Frequency (n = 827) |
Percentage |
|---|---|---|
| Acinetobacter baumannii | 320 | 38.7 |
| Pseudomonas aeruginosa | 145 | 17.5 |
| Klebsiella pneumoniae | 137 | 16.6 |
| Non-aeruginosa Pseudomonas spp. | 050 | 06.4 |
| Escherichia coli | 049 | 05.9 |
| Shewanella putrefaciens | 034 | 04.1 |
| Enterobacter spp. | 030 | 03.6 |
| Streptococcus pyogenes | 017 | 02.1 |
| Proteus spp. | 010 | 01.2 |
| Staphylococcus aureus | 018 | 2.18 |
| Streptococcus pneumoniae | 005 | 00.6 |
| Citrobacter freundii | 003 | 00.4 |
| Streptococcus spp. (other) | 003 | 00.4 |
| Serratia marcescens | 002 | 00.2 |
| Group D streptococci | 002 | 00.2 |
| Enterococci | 002 | 00.2 |
| Total | 827 | 100.0 |
Resistance in Gram-negative Organisms
Among the 231 isolates from the Enterobacteriaceae family, extended-spectrum β-lactamases (ESBLs) were produced by 42.86% of E. coli and 34.31% of Klebsiella spp.
Of 780 gram-negative organisms, 13.33% were carbapenem-resistant Enterobacteriaceae (CRE) and 38.89% were carbapenem-resistant A. baumannii (CRAB).
The distribution of A. baumannii in different seasons was analyzed. It was observed that of 320 A. baumannii, the highest number was isolated during the monsoon season followed by summer and winter seasons as shown in Figure 2. Of the 320 isolates of A. baumannii, 128 were isolated during the monsoon months of June to September. There were 85 isolates from October to January.
DISCUSSION
VAP is caused by a wide spectrum of bacterial pathogens. It may be polymicrobial and in immunocompromised hosts may be of viral or fungal etiology.3 Knowledge of predominant local pathogens and their antimicrobial susceptibility patterns assists in the selection of appropriate initial antibiotic therapy. This data served as an indicator of microbial trends and susceptibility patterns.
Gender and Age Distribution
There were 710 (66.10%) males and 364 (33.90%) females in the ratio of 2:1 in our study. The VAP incidence was higher in males than in females. Sharpe et al.27 studied 854 patients over 8 years in the ICU of Memphis, United States (US), observed a significantly higher incidence of VAP of 3.8% among males compared to 2.6% in females. In the present study, there was an increase in VAP with an increase in the age of the patient, but the correlation was not statistically significant (p value = 0.673).
A study conducted by Dey et al.15 showed that a significantly higher VAP was acquired in 46-to 60-year age-group. Old age, underlying chronic lung disease, and previous antibiotic exposures were associated with a higher risk for developing VAP reported in studies.28,29
| Organisms | Amikacin | Gentamicin | Netilmicin | Tobramycin | Ceftazidime | Cefepime | Cefoperazone–sulbactam | Piperacillin–Tazobactam | Ciprofloxacin | Imipenem | Meropenem | Colistin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acinetobacter baumannii (n = 320) | S | 17 (6%) |
32 (10%) |
51 (16%) |
45 (14%) |
9 (3%) |
6 (2%) |
19 (6%) |
29 (9%) |
14 (4%) |
11 (4%) |
11 (4%) |
320 (100%) |
| R | 303 (94%) |
288 (90%) |
269 (84%) |
275 (86%) |
311 (97%) |
314 (98%) |
301 (94%) |
291 (91%) |
306 (96%) |
309 (97%) |
309 (97%) |
0 (00%) |
|
| Pseudomonas aeruginosa (n = 145) | S | 91 (63%) |
88 (61%) |
90 (62%) |
89 (61%) |
85 (59%) |
76 (52%) |
78 (54%) |
91 (63%) |
87 (60%) |
86 (59%) |
82 (57%) |
144 (99%) |
| R | 54 (37%) |
57 (39%) |
55 (38%) |
56 (39%) |
60 (41%) |
69 (48%) |
67 (46%) |
54 (37%) |
58 (40%) |
59 (41%) |
63 (43%) |
1 (1%) |
|
| Klebsiella pneumoniae (n = 137) | S | 76 (55%) |
55 (40%) |
72 (53%) |
NA | 26 (18%) |
40 (29%) |
46 (34%) |
35 (26%) |
36 (26%) |
68 (49%) |
64 (47%) |
136 (99%) |
| R | 61 (45%) |
82 (60%) |
65 (47%) |
NA | 111 (81%) |
97 (71%) |
91 (66%) |
102 (74%) |
101 (74%) |
69 (51%) |
73 (53%) |
1 (1%) |
|
| Enterobacter spp. (n = 35) | S | 18 (55%) |
17 (52%) |
18 (55%) |
14 (42%) |
10 (30%) |
13 (39%) |
15 (45%) |
13 (39%) |
16 (48%) |
19 (58%) |
21 (64%) |
33 (100%) |
| R | 15 (45%) |
16 (48%) |
15 (45%) |
19 (57%) |
23 (70%) |
20 (61%) |
18 (55%) |
20 (61%) |
17 (52%) |
14 (42%) |
12 (36%) |
0 (00%) |
|
| Escherichia coli (n = 49) | S | 28 (57%) |
19 (39%) |
35 (71%) |
3 (6%) |
5 (10%) |
19 (39%) |
20 (41%) |
17 (35%) |
10 (21%) |
37 (76%) |
37 (76%) |
48 (98%) |
| R | 21 (43%) |
30 (61%) |
14 (29%) |
46 (94%) |
44 (90%) |
30 (61%) |
29 (59%) |
32 (65%) |
39 (81%) |
12 (24%) |
12 (24%) |
1 (02%) |
|
| Shewanella putrefaciens (n = 34) | S | 5 (15%) |
5 (15%) |
4 (12%) |
4 (12%) |
4 (12%) |
4 (12%) |
4 (12%) |
4 (12%) |
4 (12%) |
4 (12%) |
4 (12%) |
34 (100%) |
| R | 29 (85%) |
29 (85%) |
30 (88%) |
30 (88%) |
30 (88%) |
30 (88%) |
30 (88%) |
30 (88%) |
30 (88%) |
30 (88%) |
30 (88%) |
0 (00%) |
|
| Non-aeruginosa Pseudomonas spp. (n = 36) | S | 26 (52%) |
24 (48%) |
21 (42%) |
21 (42%) |
15 (30%) |
11 (22%) |
24 (48%) |
21 (42%) |
28 (56%) |
9 (18%) |
10 (20%) |
48 (96%) |
| R | 24 (48%) |
26 (52%) |
29 (58%) |
29 (58%) |
35 (70%) |
39 (78%) |
26 (52%) |
29 (58%) |
22 (44%) |
41 (82%) |
40 (80%) |
2 (04%) |
| Organism | Vancomycin | Teicoplanin | Linezolid | Clindamycin | Erythromycin | Penicillin | Gentamicin | Cefoxitin | Cefazolin | Amoxicillin-clavulanate | Ciprofloxacin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus (n = 18) | S R |
100% 0% |
100% 0% |
100% 0% |
61% 39% |
22% 78% |
11% 89% |
17% 83% |
56% 44% |
56% 44% |
56% 44% |
6% 94% |
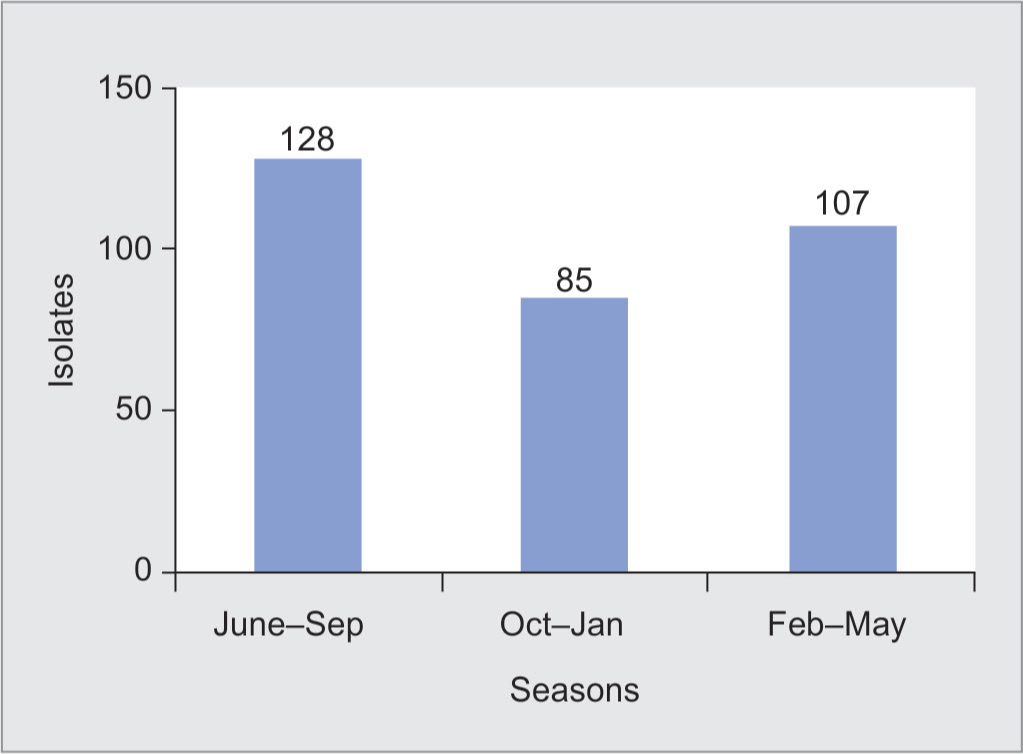
Fig. 2: Month-wise distribution of Acinetobacter isolates
Service-wise Distribution of VAP Cases
In the cases analyzed, the highest number of patients belonged to hematolymphoid, thoracic, and head and neck services.
Groeger et al.6 also found that VAP was highest in hematolymphoid malignancy than solid tumor group, while study by Park30 showed that the highest VAP was associated with solid tumor group, i.e., lung cancer cases, as lung cancer is among the most commonly diagnosed malignancies in the world and cigarette smoking has been shown to be a strong risk factor for the subsequent development of lung cancer. The cancer patients in our ICUs are on aggressive chemotherapy regimens, have low neutrophil count, and may develop drug toxicity. They are intubated for respiratory distress and stay in the ICU for a long duration on mechanical ventilation. These factors render them prone to developing VAP. However, surgical postoperative cases require shorter periods of ventilation, making them less prone to the development of VAP.
Microbiology
There were 827 bacterial isolates seen in this study. These included 780 (94.32%) gram-negative organisms and 47 (5.68%) gram-positive microorganisms.
In a meta-analysis of VAP in adults from developing countries, Arabi et al.4 reported that 41 to 92% of VAP episodes were caused by gram-negative bacilli, while 6 to 58% by gram-positive cocci. A study by Chandrakanth et al.31 in 2009 reported that-gram-negative organisms account for 89% of VAP. Chawla et al.,32 in their study also found that 87% of patients with VAP had gram-negative organisms. Quartin et al.33 from New York published the results of a multicenter trial from October 2004 through January 2010. In this, 63.4% of identified organisms were gram-positives with 42.7% being MRSA. The gram-negatives constituted 36.6%. Worldwide data indicate that in Western countries gram-positive organisms predominate. Potential reasons include the use of indwelling catheters, local environmental conditions, and the administration of specific antibiotic agents, especially as prophylaxis. As per Indian studies, gram-negative organisms are the major cause of VAP. This can be linked with colonization of the gut and exposure to antimicrobials. The critically ill patients get colonized exogenously or endogenously with hospital flora within 24–48 hours of hospitalization and the oral flora shifts to a predominance of hospital microbial flora, i.e., aerobic gram-negative pathogens. Pulmonary aspiration of these oropharyngeal contents increases the risk for infection. Also, critically ill patients are on broad-spectrum empirical antibiotics, which cause selection pressures on these colonizers for the emergence of resistant strains of gram-negative pathogens.34,35 The presence of an endotracheal tube in ventilated patients impairs mucociliary clearance and disrupts the cough reflex, thus promoting the accumulation of tracheobronchial secretions and increasing the risk of pneumonia.36 In addition, insertion of an endotracheal tube could produce injury and inoculate these endogenous oropharyngeal bacteria, such as A. baumannii and P. aeruginosa in the lower airway tract and pulmonary aspiration of these oropharyngeal contents increases the risk of airway colonization and infection.35 The formation of a biofilm on the surface of the endotracheal tube is related to the pathogenesis of VAP.36-39
The common organisms isolated from cases with VAP in our study were A. baumannii followed by P. aeruginosa and K. pneumoniae. Al-Dorzi et al.13 from Saudi Arabia during 2003 to 2008 reported that A. baumannii was the most commonly cultured microorganism (19%), causing VAP. In a prospective study conducted by Joseph et al.,14 in 2006–2007, A. baumannii (21.3%) and P. aeruginosa (21.3%) were the most common gram-negative bacteria associated with VAP and S. aureus (14.9%) was the most common gram-positive organism. Similar findings were reported by Dey et al.,15 Rajasekhar et al.,40 and Goel et al.,41 where A. baumannii was the commonest organism causing VAP followed by P. aeruginosa.
Colonization of the respiratory tract with Acinetobacter spp., Pseudomonas spp., and MRSA may have originated from endogenous sources, such as the oropharynx or the stomach, or from exogenous sources, such as contaminated respiratory instruments, infective aerosols from the ICU environment, and contaminated hands and apparel of the healthcare workers. These act as vehicles of transmission. Handwashing is the single most effective measure of preventing transmission. Also, many of our VAP patients had risk factors for acquiring multidrug-resistant organisms (MDROs), such as advanced age, underlying immunosuppression— chronic renal failure, diabetes mellitus, acquired immunodeficiency syndrome, and on immunosuppressants—exposure to broad-spectrum antibiotics in preceding 3 months, increased severity of illness, previous multiple hospitalizations, and prolonged duration of invasive mechanical ventilation.2,3,42
A. baumannii is omnipresent in the environment and can survive on nonliving inert environmental surfaces. It is frequently isolated from hospital water systems along with other water organisms like P. aeruginosa, Stenotrophomonas maltophilia, and Legionella pneumophila. The emergence of A. baumannii as an important cause of nosocomial infections is favored by three major factors, like resistance to drying, disinfectants, and antimicrobial agents.41 Its prolonged survival on inanimate objects in the hospital environment and hospital water can be a constant source of this organism. It may be carried on the hands of healthcare workers and patients, and spreads readily from healthcare workers to patients or from patients to patients. This can result in outbreaks in the unit which are difficult to control.43,44 It is reported that Acinetobacter spp. has the ability to acquire resistance determinants more effectively than other bacteria. Innate colistin resistance is common in certain Acinetobacter species, such as Acinetobacter junii.45 The majority of the A. baumannii isolates were observed during July through September. This may be attributed to the high environmental temperature and high level of humidity during the monsoon with resultant prolonged survival of organisms in the environment. Siau et al.,46 in Hong Kong observed a seasonal variation in the isolation of Acinetobacter spp., corresponding to a peak period from July through October (the hot, humid season), during which increased numbers of Acinetobacter spp. were isolated.
Antimicrobial Susceptibility Patterns
It was observed in our study that antibiotic resistance in gram-negative organisms was on the rise in general. Resistance to aminoglycosides was high at 94% to amikacin and 90% to gentamicin. More than 90% of the strains were resistant to the tested β-lactam-β-lactamase inhibitor (BLBLI) combinations namely cefoperazone–sulbactam and piperacillin–tazobactam. However, all A. baumannii isolates were susceptible to colistin.
Sievert et al.47 reported data from US hospitals in 2009 and 2010 and found that 63.4% of Acinetobacter isolates were resistant to aminoglycosides and piperacillin–tazobactam and 61.2% to the carbapenems. Balkhy et al.48 studied 248 isolates of A. baumannii and found that 83 to 88% isolates were resistant to aminoglycoside group of antimicrobials, 60–71% to carbapenems like imipenem and meropenem, 86–89% to third-generation cephalosporins, and 86% to the fluoroquinolones. In a multicenter study from Turkey hospitals,49 90.03% of Acinetobacter were resistant to piperacillin, 87.54% to ciprofloxacin, and 78.29% to meropenem. Moreira et al. from Brazil50 found that 80.9% of isolates of A. baumannii were resistant to carbapenems. Among the studies reported from India, Goel et al.41 found that 92.59% of Acinetobacter isolates were resistant to amikacin, 88.89% to meropenem, 85.18% to ceftazidime, and 37.04% to piperacillin–tazobactam. Gupta et al.10 conducted a prospective study in the general ICU in 2011 and reported that 50% of A. baumannii were carbapenem resistant. This shows that the resistance pattern shown by Acinetobacter isolates in our study was high compared to Western studies and was comparable with Indian studies. In the present study, resistance to the tested BLBLI combinations namely cefoperazone–sulbactam and piperacillin–tazobactam was higher than in other Indian studies. This could be because of the empirical usage of higher antibiotics in cancer patients.
The susceptibility of P. aeruginosa isolates was highest to colistin (99%). Resistance to amikacin and piperacillin–tazobactam was 37%. Resistance to ceftazidime and ciprofloxacin was 41% and to cefoperazone–sulbactam was 46 and 42% to carbapenems. Balkhy et al.48 found that P. aeruginosa had 31% resistance to carbapenems, 27–28% to third-generation cephalosporins, and 13–25% to aminoglycosides. Sievert et al.47 reported that
P. aeruginosa isolates showed 11.3% resistance to amikacin, 19.1% to piperacillin–tazobactam, 28.4% to cefepime and ceftazidime, 32.7% to ciprofloxacin/levofloxacin, and 30.2% to imipenem/meropenem. In Indian studies, Goel et al.41 found that P. aeruginosa showed 100% resistance to gentamicin, 82.35% to amikacin and ciprofloxacin, 47.06% to imipenem, 35.29% to ceftazidime, and 23.53% to piperacillin–tazobactam. The levels of resistance shown by P. aeruginosa in this study were high compared to the Western literature. In comparison to other Indian studies, higher resistance was observed to the BLBLI combination piperacillin–tazobactam.
Our study shows that there was a high level of resistance to BLBLIs and carbapenems in the case of K. pneumoniae. Haeili et al.51 in a retrospective study observed that 20.4% of K. pneumoniae were resistant to carbapenems and 50% were resistant to amikacin and gentamicin. Sievert et al.47 reported that K. pneumoniae isolates showed 23.8% resistance to cefepime, cefotaxime, ceftazidime, and ceftriaxone, and 11.2% resistance to imipenem and meropenem.
All S. aureus isolates were susceptible to vancomycin, teicoplanin, and linezolid, and 44.45% were MRSA which showed 100% resistance to ciprofloxacin and gentamicin. Balkhy et al.48 found that all isolates of S. aureus were susceptible to vancomycin and 42% of isolates were methicillin-resistant strains.
The findings in the current study were consistent with these studies. It was observed that multidrug resistance is increasing gradually in hospital isolates, particularly in case of Acinetobacter spp., P. aeruginosa, K. pneumoniae, and S. aureus. A number of studies in the literature also indicate a gradual increase in the emergence of antibiotic-resistant microorganisms in VAP patients.
Studies from Indian hospitals from International Nosocomial Infection Control Consortium have shown that MDR P. aeruginosa was the most common bacterial isolate in VAP patients,52 which inevitably resulted in the increased use of carbapenems that might have contributed to the emergence of MDR nonfermentative gram-negative bacilli, mainly A. baumannii. In this study, the increase in the incidence of VAP due to MDR A. baumannii again resulted in increased clinical use of carbapenems and polymyxins like colistin. A study conducted by Mulin et al.53 showed the association of third-generation cephalosporins with colonization and infection with MDROs Acinetobacter spp. Risk factors for VAP were commonly prevalent in our patients, making them more susceptible to acquiring VAP. Multidrug resistance is defined as resistance to either three or four classes of antimicrobial agents, including penicillins, cephalosporins, carbapenems, fluoroquinolones, and aminoglycosides. MDR P. aeruginosa and gram-negative bacteria producing ESBL enzymes have created treatment challenges for critical care clinicians, leaving the carbapenem class of antimicrobial agents as the last choice to treat patients with these resistant infections. Prevention and control of MDROs in critical care units is a major task. There are very few antimicrobials in the pipeline and there is an urgent need to change the approach from “treatment” to “prevention.” Robust antimicrobial stewardship programs involving pharmacists, physicians, and other healthcare providers to optimize antibiotic selection, dose, and duration thereby increasing the efficacy in targeting causative pathogens for the best clinical outcome are the way forward.
CONCLUSION
MDROs constituted 87.5% of all gram-negative bacilli, of which A. baumannii was the most common pathogen associated with VAP in the current study and had a very high (84–97%) resistance rate to all tested antimicrobials except colistin. Knowledge of locally prevalent organisms and their susceptibility patterns can serve as a guide for optimal empirical antibiotic therapy of VAP and also help reduce the emergence of MDR strains in our setting.
Limitations of the Study
The variables, such as the date of admission to ICU, reason, and duration of mechanical ventilation, comorbidities, surgical procedures, and progress of patient, could not be assessed.
ACKNOWLEDGMENTS
Dr. Rohini S. Kelkar, Dr. Vivek Bhat , Dr. Sanjay Biswas, Dr. Prashant Mule, Dr. Amruta Tikhile and the entire microbiology laboratory staff.
ORCID
Aarti Sangale https://orcid.org/0000-0001-8687-6000
Vivek Bhat https://orcid.org/0000-0001-5085-2007
Rohini Kelkar https://orcid.org/0000-0001-9975-0239
Sanjay Biswas https://orcid.org/0000-0002-9802-0848
REFERENCES
1. Lassen HCA. The epidemic of poliomyelitis in Copenhagen, 1952. Proc R Soc Med 1954;47(1):67-71.
2. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer, et al. American Thoracic Society: Infectious Diseases Society of America. Guidelines for the management of adults with hospital acquired, ventilator associated and healthcare associated pneumonia. Am J Respir Crit Care Med 2005;171(4):388-416. DOI: 10.1164/rccm.200405-644ST.
3. Chastre J, Fagon JY. Ventilator associated pneumonia. Am J Respir Crit Care Med 2002;165(7):867-903. DOI: 10.1164/ajrccm.165.7. 2105078.
4. Arabi Y, Al-Shirawi N, Memish Z, Anzueto A. Ventilator-associated pneumonia in adults in developing countries: a systematic review. Int J Infect Dis 2008;12(5):505-512. DOI: 10.1016/j.ijid.2008.02.010.
5. Kalanuria AA, Zai W, Mirski M. Ventilator associated pneumonia in the ICU. J Crit Care 2014;18(2):208. DOI: 10.1186/cc13775.
6. Groeger JS, White P. Outcome for cancer patients requiring Mechanical Ventilation. J Clin Oncol 1999;17(3):991-997. DOI: 10.1200/JCO.1999.17.3.991.
7. Schapira D, Studnicki J, Bradham D, Wolff P, Jarrette A. Intensive care, survival, and expense of treating critically ill cancer patients. JAMA 1993;269:783-786. DOI: 10.1001/jama.1993.03500060083036.
8. Davis KA. Ventilator associated pneumonia: a review. J Intensive Care Med 2006;21:211-226. DOI: 10.1177/0885066606288837.
9. Rakshit P, Nagar VS, Deshpande AK. Incidence, clinical outcome and risk stratification of ventilator-associated pneumonia: A prospective cohort study. Indian J Crit Care Med 2005;9(4):211-216. DOI: 10.4103/0972-5229.19761.
10. Gupta A, Agrawal A, Mehrotra S, Singh A, Malik S, Khanna A. Incidence, risk stratification, antibiogram of pathogens isolated and clinical outcome of Ventilator associated pneumonia. Indian J Crit Care Med 2011;15(2):96-101. DOI: 10.4103/0972-5229.83015.
11. Bonell A, Azarrafiy R, Huong VTL, Viet TL, Phu VD, Dat VQ, et al. A systematic review and meta-analysis of ventilator-associated pneumonia in adults in Asia: an analysis of national income level on incidence and etiology. Clin Infect Dis 2019;68(3):511-518. DOI: 10.1093/cid/ciy543.
12. Papazian L, Bregeon F, Thirion X, Gregoire R, Saux P, Denis JP, et al. Effect of ventilator associated pneumonia on mortality and morbidity. Am J Respir Crit Care Med 1996;154(1):91-97. DOI: 10.1164/ajrccm.154.1.8680705.
13. Balkhy HH, El-Saed A, Maghraby R, Al-Dorzi HM, Khan R, Rishu AH, et al. Multi drug-resistant versus sensitive Acinetobacter baumannii ventilator associated pneumonia at a tertiary care centre: characteristics, microbiology and outcomes. Am J Respir Crit Care Med 2012;18:50-53. DOI: 10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A6076.
14. Joseph N, Sistla S, Dutta T, Badhe A, Parija S. Ventilator associated pneumonia in a tertiary care hospital in India: incidence and risk factors. J Infect Dev Ctries 2009;3(10):771-777. DOI: 10.3855/jidc.396.
15. Dey A, Bairy I. Incidence of multidrug-resistant organisms causing ventilator associated pneumonia in a tertiary care hospital: a nine months' prospective study. Ann Thorac Med 2007;2(2):52-57. DOI: 10.4103/1817-1737.32230.
16. Wałaszek M, Różańska A, Wałaszek MZ, Wójkowska-Mach J, The Polish Society of Hospital Infections Team. Epidemiology of ventilator-associated pneumonia, microbiological diagnostics and the length of antimicrobial treatment in the Polish Intensive Care Units in the years 2013-2015. BMC Infect Dis 2018;18:308. DOI: 10.1186/s12879-018-3212-8.
17. Gadani H, Vyas A, Kar AK. A study of ventilator-associated pneumonia: Incidence, outcome, risk factors and measures to be taken for prevention. Indian J Anaesth 2010;54(6):535-540. DOI: 10.4103/0019-5049.72643.
18. Rosenthal VD, Maki DG, Salomao R, Moreno CÁ, Mehta Y, Higuera F, et al. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med 2006;145(8):582-591. DOI: 10.7326/0003-4819-145-8-200610170-00007.
19. Koenig SM, Truwit JD. Ventilator associated pneumonia: diagnosis, treatment and prevention, Clin Microbiol Rev 2006;19(4):637-657. DOI: 10.1128/CMR.00051-05.
20. Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short course empiric antibiotic therapy for patients with pulmonary infilterates in the intensive care units: a proposed solution for indiscriminate antibiotic prescription. AM J Respir Crit Care Med 2009;162(2 Pt 1): 505-511. DOI: 10.1164/ajrccm.162.2.9909095.
21. Estella A, Monge MI, Perez Fontaina L, Sainz de Baranda A, Gala MJ, Moreno E. Bronchoalveolar lavage for diagnosing pneumonia in mechanically ventilated patients. Med Intensiva 2008;32(9):419-423. DOI: 10.1016/s0210-5691(08)75718-8.
22. Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: Its relevance to developing effective strategies for prevention. Respir Care 2005;50(6):725-739.
23. Kollef MH. What is ventilator-associated pneumonia and why is it important? Respir Care 2005;50:714-721.
24. Tille PM., Forbes BA. Bailey & Scott's diagnostic microbiology. 13th ed. St. Louis, Missouri: Elsevier; 2014.
25. Winn W Jr, Allen S, Janda W, Koneman E, Procop G, Schreckenberger P, et al. Koneman's colour atlas and textbook of diagnostic microbiology. 6th ed. Lippincot; 2006.
26. Wu CL, Yang DI, Wang NY, Kuo HT, Chen PZ. Quantitative culture of endotracheal aspirates in the diagnosis of ventilator-associated pneumonia in patients with treatment failure. Chest 2002;122(2):662-668. DOI: 10.1378/chest.122.2.662.
27. Sharpe JP, Magnotti LJ, Weinberg JA, Brocker JA, Schroeppel TJ, Zarzaur BL, et al. Gender disparity in ventilator-associated pneumonia following trauma: identifying risk factors for mortality. J Trauma Acute Care Surg 2014;77(1):161-165. DOI: 10.1097/TA.0000000000000251.
28. Torres A, Carlet J. Ventilator-associated pneumonia. European Task Force on ventilator-associated pneumonia. Eur Respir J 2001;17(5):1034-1045. DOI: 10.1183/09031936.01.17510340.
29. O'Grady NP, Murray PR, Ames N. Preventing ventilator-associated pneumonia: does the evidence support the practice? JAMA 2012;307(23):2534-2539. DOI: 10.1001/jama.2012.6445.
30. Park SA, Cho SS, Kwak GJ. Factors influencing ventilator-associated pneumonia in cancer patients. Asian Pac J Cancer Prev 2014;15(14):5787-5791. DOI: 10.7314/apjcp.2014.15.14.5787.
31. Chandrakanth C, Anushree, Vinod A. Incidence of ventilator associated pneumonia. Int J Med Clin Res 2010;1(2):11-13. DOI: 10.9735/0976-5530.1.2.11-13.
32. Chawla R. Epidemiology, etiology and diagnosis of hospital acquired pneumonia and ventilator associated pneumonia in Asian countries. Am J Infect Control 2008;36(4 Suppl.):93-100. DOI: 10.1016/j.ajic.2007.05.011.
33. Quartin AA, Kett DH, Scerpella EG, Huang DB. A comparison of microbiology and demographics among patients with healthcare-associated, hospital acquired, and ventilator-associated pneumonia: a retrospective analysis of 1184 patients from a large, international study. BMC Infect Dis 2013;13:561-564. DOI: 10.1186/1471-2334-13-561.
34. Rahal JJ, Urban C, Segal-Maurer S. Nosocomial antibiotic resistance in multiple Gram negative species: experience at one hospital with squeezing the resistance balloon at multiple sites. Clin Infect Dis 2002;34(4):499-503. DOI: 10.1086/338639.
35. Gottesman BS, Carmeli Y, Shitrit P, Chowers M. Impact of quinolone restriction on resistance patterns of Escherichia coli isolated from urine by culture in a community setting. Clin Infect Dis 2009;49(6):869-875. DOI: 10.1086/605530.
36. Craven DE, Steger KA. Epidemiology of nosocomial pneumonia. New perspectives on an old disease. Chest 1995;108(2 Suppl.):1S-16S. DOI: 10.1378/chest.108.2_supplement.1s.
37. Rello J, Sonora R, Jubert P, Artigas A, Rue M, Valles J. Pneumonia in intubated patients: role of respiratory airway care. Am J Respir Crit Care Med 1996;154(1):111-115. DOI: 10.1164/ajrccm.154.1.8680665.
38. Donlan RM. Role of biofilms in antimicrobial resistance. Asaio J 2000;46(6):S47-S52. DOI: 10.1097/00002480-200011000-00037.
39. Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet 2001;358(9276):135-138. DOI: 10.1016/s0140-6736(01)05321-1.
40. Rajasekhar T, Anuradha K, Suhasini T, Lakshmi V. The role of quantitative cultures of non bronchoscopic samples in ventilator associated pneumonia. IJMM 2006;24(2):107-113. DOI: 10.4103/0255-0857.25226.
41. Goel V, Hogade SA, Karadesai SG. Ventilator associated pneumonia in a medical intensive care unit: microbiological aetiology, susceptibility patterns of isolated organisms and outcome. Indian J Anasth 2012;56(6):558-562. DOI: 10.4103/0019-5049.104575.
42. Divatia JV, Pulinilkunnathil JG, Myatra SN. Nosocomial infections and ventilator-associated pneumonia in cancer patients. Oncol Crit Care 2019;1419-1439. DOI: 10.1007/978-3-319-74588-6_125.
43. Baran G, Erbay A, Bodur H, Onguru P, Akinci E, Balaban N, et al. Risk factors for nosocomial imipenem-resistant Acinetobacter baumannii infections. Int J Infect Dis 2008;12(1):16-21. DOI: 10.1016/j.ijid.2007.03.005.
44. Neonakis IK, Spandidos DA, Petinaki E. Confronting multidrug-resistant Acinetobacter baumannii: a review. Int J Antimicrob Agents 2011;37(2):102-109. DOI: 10.1016/j.ijantimicag.2010.10.014.
45. Rice LB. Challenges in identifying new antimicrobial agents effective for treating infections with Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis 2006;43(2):S100-S105. DOI: 10.1086/504487.
46. Siau H, Yuen KY, Wong SSY, Ho PL, Luk WK. The epidemiology of Acinetobacter infections in Hong Kong. J Med Microbiol 1996;44:340-347. DOI: 10.1099/00222615-44-5-340.
47. Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol 2013;34(1):1-14. DOI: 10.1086/668770.
48. Balkhy HH, El-Saed A, Maghraby R, Al-Dorzi HM, Khan R, Rishu AH, et al. Drug-resistant ventilator associated pneumonia in a tertiary care hospital in Saudi Arabia. Ann Thorac Med 2014;9(2):104-111. DOI: 10.4103/1817-1737.128858.
49. ÇiÇek AÇ, Düzgün AÖ, Saral A, Kayman T, Çizmeci Z, Balci PÖ, et al. Detection of class 1 integron in Acinetobacter baumannii isolates collected from nine hospitals in Turkey. Asian Pac J Trop Biomed 2013;3(9):743-747. DOI: 10.1016/S2221-1691(13)60149-5.
50. Moreira MR, Guimaraes MP, Rodrigues AA, Gontijo Filho PP. Antimicrobial use and bacterial resistance in VAP. Rev Soc Bras Med Trop 2013;46(1):39-44. DOI: 10.1590/0037-868216722013.
51. Haeili M, Ghodousi A, Nomanpour B, Omrani M, Faizabadi MM. Drug resistance patterns of bacteria isolated from patients with nosocomial pneumonia at Tehran hospitals during 2009-2011. J Infect Dev Ctries 2013;7(4):312-317. DOI: 10.3855/jidc.2604.
52. Mehta Y, Jaggi N, Rosenthal VD, Rodrigues C, Todi SK, Saini N, et al. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in 21 adult intensive-care units from 10 cities in India: findings of the International Nosocomial Infection Control Consortium (INICC). Epidemiol Infect 2013;141(12):2483-2491. DOI: 10.1017/S0950268813000381.
53. Mulin B, Talon D, Viel JF, Vincent C, Leprat R, Thouverez M, et al. Risk factors for nosocomial colonization with multiresistant Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis 1995;14(7):569-576. DOI: 10.1007/BF01690727.
________________________
© The Author(s). 2021 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.