LETTER TO EDITOR |
https://doi.org/10.5005/jp-journals-10071-23794 |
Second-degree Heart Block Caused by Itolizumab-induced Infusion Reaction in COVID-19
1,3Department of Anaesthesiology, All India Institute of Medical Sciences, Patna, Bihar, India
2,4Department of Trauma and Emergency, All India Institute of Medical Sciences, Patna, Bihar, India
5Department of Cardiology, All India Institute of Medical Sciences, Patna, Bihar, India
Corresponding Author: Abhyuday Kumar, Department of Anaesthesiology, All India Institute of Medical Sciences, Patna, Bihar, India, Phone: +91 9013512403, e-mail: drabhyu@gmail.com
How to cite this article: Kumar A, Kumar N, Lenin D, Kumar A, Ahmad S. Second-degree Heart Block Caused by Itolizumab-induced Infusion Reaction in COVID-19. Indian J Crit Care Med 2021;25(4):474–475.
Source of support: Nil
Conflict of interest: None
Sir,
Itolizumab, an anti-CD6 humanized IgG1 monoclonal antibody, binds to domain-1 of CD-6 that is responsible for priming, activation, and differentiation of T-cells.[1] It significantly reduces T-cell proliferation along with substantial downregulation of the production of cytokines/chemokines.1 It was approved for moderate to severe chronic plaque psoriasis in 2013. However, it has recently been approved by the Drug Controller General of India for emergency use in India for the treatment of cytokine release syndrome in moderate to severe acute respiratory distress syndrome patients due to COVID-19.2 Here, we report a case of life-threatening infusion-related hypersensitivity reaction of itolizumab.
A 65-year-old male COVID-19 patient got admitted to the intensive care unit (ICU) with complaints of shortness of breath and cough without any history of known disease. However, the baseline electrocardiogram (ECG) done in the ICU was suggestive of left bundle branch block (LBBB) (Fig. 1A). The patient was supported through noninvasive ventilation (NIV) and was started on remdesivir, dexamethasone, low-molecular-weight heparin, antibiotics, and other supportive treatment as per our institutional standard protocol. The patient was maintaining on continuous positive airway pressure mode of NIV with a fraction of inspired oxygen (FiO2) of 0.5 on the third day of ICU admission. Among the laboratory markers, the total leucocyte counts were raised (12,000/μL) with decreased lymphocytes (3.2%) and increased inflammatory markers (CRP, 320 mg/L; D dimer >20 μg/mL; LDH, 1694 U/L; IL6, 329 pg/mL). Serum electrolytes, renal function tests, liver function tests, and arterial blood gases were within acceptable limits. The patient was hemodynamically stable with a respiratory rate of 30 to 35/minute and a PO2/FiO2 ratio of 140. After taking informed written consent, inj. itolizumab was planned in this patient because of the increasing severity of the disease along with increased inflammatory markers. Inj. hydrocortisone 100 mg IV and inj. pheniramine 30 mg IV were given 30 minutes before itolizumab infusion. And 100 mg of itolizumab (Alzumab-L, Biocon Biologics) was diluted to 250 mL with normal saline and was started at 25 mL/hour. After about 20 minutes of infusion, the patient started complaining of shivering, sweating, and impending doom. The patient had sudden bronchospasm, and oxygen saturation dropped to 90%. ECG showed second-degree AV nodal block with an increased blood pressure of 180/110 mm Hg (Fig. 1B). The drug was immediately withdrawn and the patient was given a repeat dose of hydrocortisone and pheniramine along with other supportive measures. After sometime patients became alert and their respiratory symptoms were relieved. However, the second-degree heart block in ECG was persistent. ECHO was normal and troponin I was within normal limits while there was a slight increase in CPK-MB. The patient was observed closely and the ECG reverted to its previous state only after 24 hours. The patient was weaned from the ventilator in due course of time and put on face mask on the eighth day of stay.
Most infusion reactions related to monoclonal antibodies are IgE mediated and are mild (grade 1 or 2) in nature.3 The incidence of severe (grade 3 or 4) reactions is generally low. The reported infusion-related reactions to itolizumab are chills/rigors (common), nausea, flushing, urticaria, cough, hypersensitivity, pruritus, rash, wheezing, dyspnea, oxygen desaturation, dizziness, headache, and hypertension. In our case, itolizumab infusion leads to a grade 4 reaction causing a persistent second-degree heart block for about 24 hours. Among the monoclonal antibodies, rituximab is most notorious for causing infusion reactions.4 There are only a few reports of cardiac arrhythmias (monomorphic VT, supraventricular tachycardia, trigeminy, and irregular pulse) during therapeutic infusion of rituximab,5 and there is no reported case of cardiac arrhythmia during itolizumab infusion. In our case, the patient was having LBBB and was on a QT prolonging drug (remdesivir), which might be a predisposing factor for the occurrence of second-degree heart block during infusion reaction. Premedications (e.g., antipyretics, antihistamines, and steroids) are recommended before the administration of some chemotherapeutic agents and monoclonal antibodies. These drugs should never be given as IV bolus and should always be given slowly in an infusion. Baseline assessments including vital signs and cognition should be documented carefully before the start of treatment and all the emergency equipment and drugs should be kept ready. Grade 3 and 4 reactions should be managed promptly with epinephrine, antihistaminics, and steroids along with other symptomatic supportive measures. As itolizumab is approved for emergency use in COVID-19, risk-benefit ratio should be assessed before prescribing this and should be explained before taking consent for infusion.
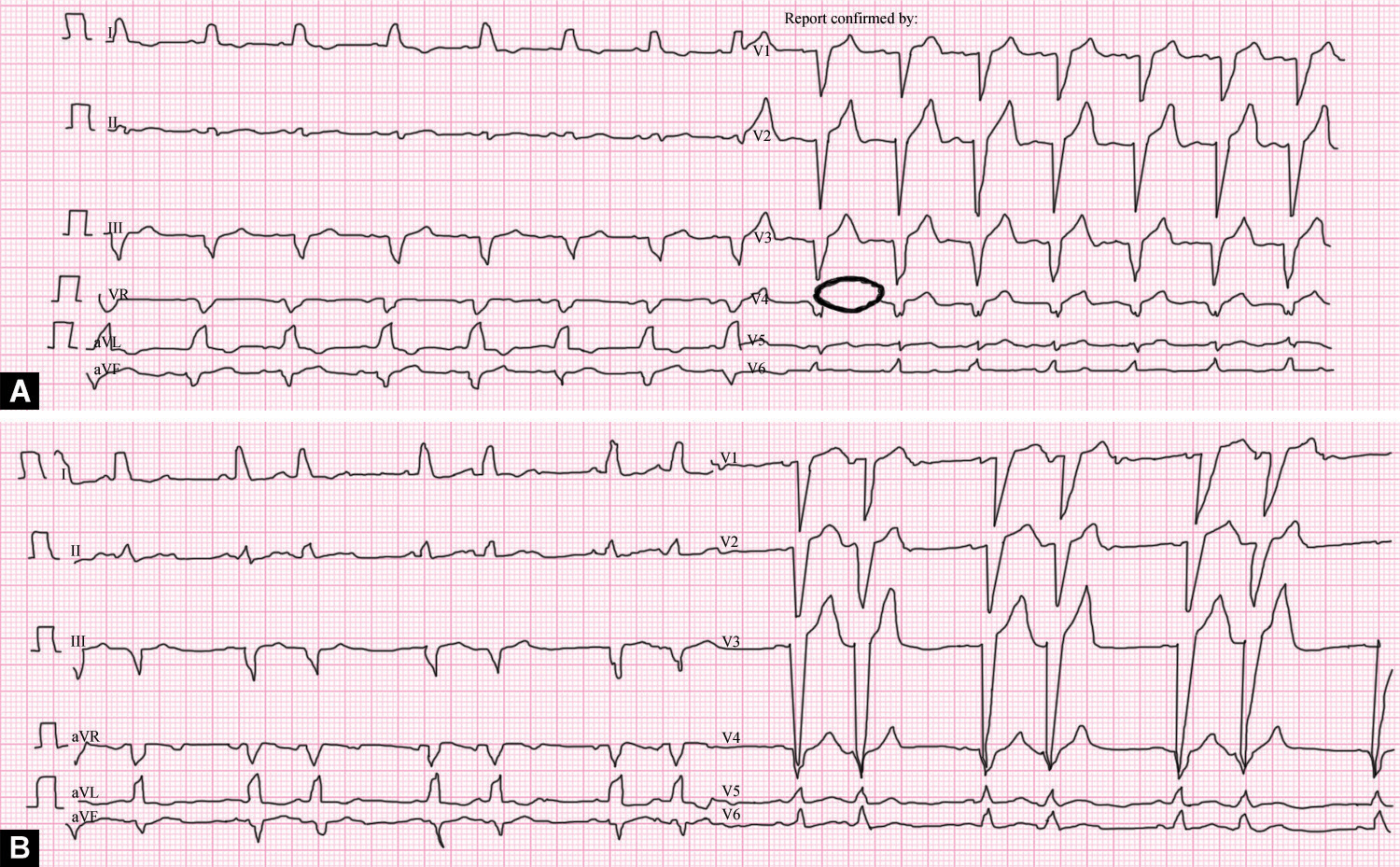
Figs 1A and B: (A) Baseline ECG showing LBBB; (B) ECG showing second-degree AV nodal block after infusion reaction
The patient provided written informed consent for the publication.
ORCIDS
Abhyuday Kumar https://orcid.org/0000-0002-9247-6713
Neeraj Kumar https://orcid.org/0000-0002-9161-7000
Dharani Lenin https://orcid.org/0000-0001-8873-8525
Amarjeet Kumar https://orcid.org/0000-0002-4272-5750
Shaheen Ahmad https://orcid.org/0000-0002-6316-3320
REFERENCES
1. Nair P, Melarkode R, Rajkumar D, Montero E. CD6 synergistic co-stimulation promoting proinflammatory response is modulated without interfering with the activated leucocyte cell adhesion molecule interaction. Clin Exp Immunol 2010;162(1):116-130. DOI: 10.1111/j.1365-2249.2010.04235.x.
2. Ministry of Health and Family Welfare, India. DCGI gives Nod for restricted emergency use to Itolizumab for moderate to severe COVID-19 patients. Press release. Available from: https://pib.gov.in/PressReleasePage.aspx?PRID=1637926. Accessed 22 December 2020.
3. Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist 2007;12(5):601-609. DOI: 10.1634/theoncologist.12-5-601.
4. Vogel WH. Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs 2010;14(2):E10-E21. DOI: 10.1188/10.CJON.E10-E21.
5. Poterucha JT, Westberg M, Nerheim P, Lovell JP. Rituximab-induced polymorphic ventricular tachycardia. Tex Heart Inst J 2010;37(2): 218-220. PMID: 20401299. PMCID: PMC2851419.
________________________
© The Author(s). 2021 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.