ORIGINAL ARTICLE |
https://doi.org/10.5005/jp-journals-10071-23909 |
Off-label Drug Prescription Pattern and Related Adverse Drug Reactions in the Medical Intensive Care Unit
1,3–5Department of Clinical Pharmacy, BVDU Poona College of Pharmacy, Pune, Maharashtra, India
2Department of Internal Medicine, Bharati Hospital, Pune, Maharashtra, India
6Department of Critical Care Medicine, Bharati Vidyapeeth [Deemed to be University] Medical College, Pune, Maharashtra, India
Corresponding Author: Asawari Raut, Department of Clinical Pharmacy, BVDU Poona College of Pharmacy, Pune, Maharashtra, India, Phone: +91 8805058493, e-mail: asawari.raut@gmail.com
How to cite this article: Raut A, Krishna K, Adake U, Sharma AA, Thomas A, Shah J. Off-label Drug Prescription Pattern and Related Adverse Drug Reactions in the Medical Intensive Care Unit. Indian J Crit Care Med 2021;25(8):872–877.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Introduction: The utilization of prescription drugs as off-label is common. While this practice can be beneficial to some patients, it can raise a safety concern when scientific evidence is lacking; hence, this study was conducted to evaluate the off-label drug consumption and its adverse drug reactions (ADRs) in the medical intensive care unit (ICU).
Materials and methods: In the prospective cohort study conducted for a duration of 6 months, data pertaining to ICU patients’ (age ≥18 years) demography, diagnosis, treatment, and laboratory investigation were collected to assess for off-label use as well as the strength of evidence and the occurrence of ADRs by using MICROMEDEX 2017 version (Healthcare Series Thomson Reuter, Greenwood, CO).
Results: Of total 3574 drugs prescribed, 1453 (41%) were off-label indications and 65 (1.81%) were off-label dose. On the evaluation of off-label indication use, 1279 (88%) were evidence-based and 174 (12%) were low/no evidence-based medications (EBMs); 59 (91%) were evidence-based and 6 (9%) were low/no EBMs for off-label dose. Most commonly prescribed evidence-based off-label drug belonged to the gastrointestinal class while low/no evidence drugs were mostly of anti-infective class. A total of 383 ADRs were identified and 139 (36.2%) were implicated due to off-label medications, of which ADRs with evidence off-label medications (87.8%) were higher than low/no evidence off-label medication (12.2%) (P < 0.001).
Conclusion: Widespread presence of off-label use was observed in medical ICU. Although incidence of ADRs was similar to the FDA-approved use, ongoing monitoring of such practice is needed.
Keywords: Adverse drug reactions, Cohort study, FDA-approved drug, Intensive care unit, Off-label drug prescription.
INTRODUCTION
“Off-label drug use is an unapproved use of an approved drug for any disease or medical condition.”1 Most physicians, without specific guidance, deduce off-label prescribing as appropriate and are convinced that the benefits outweigh the risk. However, the awareness about the risk of side effects and unevaluated efficacy seems to be minimal.2 Off-label prescribing decisions are generally derived from clinical and research-based information contingent on evidence-based medication (EBM). “The EBM concept represents the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients.” This very concept motivates some physicians to use off-label medications when it is deemed rational.3 Emergency care provided in the ICU is frequently rapid, complex, and necessitates urgent high-risk decisions with insufficient data.4 This may contribute to the higher rate of off-label drug use than somewhere else.4 This raises a concern about the patient’s safety and potential of adverse drug reactions. Comprehensive studies on off-label drug use and its adverse drug reactions (ADRs) have been conducted on children but the adults are yet to be evaluated. Also, there are not many studies available in the Indian scenario. It is therefore essential to assess the effect of this practice on the safety critical patients. Accordingly, the present study was conducted to find out the incidence of ADRs due to off-label drugs use in medical intensive care unit (ICU) patients.
MATERIALS AND METHODS
A prospective cohort study was conducted for a duration of 6 months. A total of 360 patients were involved as per the inclusion criteria limited to all adult patients admitted to ICU; there were no specific exclusion criteria as inclusion was highly restricted. Institutional ethics committee approval was obtained prior to initiation of the study (IEC ref: BVDU/MC/E4). Modified patient profile form was developed to collect data of individual patient according to the inclusion criteria of all ICU patients above 18 years. Data were obtained through patient’s charts by Doctor of Pharmacy students, which were evaluated for the drug category, dosage, route of administration, off-label indication with its strength of evidence, and ADRs to assess its causality and severity.
EVALUATION OF OFF-LABEL DRUG USE
All the medications prescribed to the patient during their ICU stay were included in the study. Pharmacological treatment information including drug indication, dosage, route of administration, frequency, and duration were collected. Also patients’ demographic details were collected. All prescribed drugs were then assessed to verify whether they were given for off-label indications and thereupon, these drugs were then categorized in accordance with the reason for considering them as off-label: dose, indication, patient population, and route of administration.
The internationally acknowledged drug information database “MICROMEDEX SOLUTION 2017 version (Healthcare Series Thomson Reuter, Greenwood, CO)” was used to evaluate the strength of the evidence supporting each off-label indication. Category A and B was considered as off-label evidence drug and category C and D was considered as off-label low/no evidence drug.5
| Strength of evidence5 | |
|---|---|
| Category A | Category A evidence is based on data derived from meta-analyses of randomized controlled trials with homogeneity about the directions and degrees of results between individual studies. Multiple, well-done randomized clinical trials involving large numbers of patients. |
| Category B | Category B evidence is based on data derived from meta-analyses of randomized controlled trials with conflicting conclusions regarding the directions and degrees of results between individual studies. Randomized controlled trials that involved small numbers of patients or had significant methodological flaws (e.g., bias, drop-out rate, and flawed analysis). Nonrandomized studies (e.g., cohort studies, case–control studies, and observational studies). |
| Category C | Category C evidence is based on data derived from expert opinion or consensus, case reports, or case series. |
| Category D | No evidence |
EVALUATION OF ADRs
All ICU patients were assessed for ADR identification every day during ward rounds and documented in the patients’ chart. The occurrence of an adverse event (AE) was not considered. “An ADR is a response to a drug which is noxious and unintended, and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of diseases or for modification of physiological function”5 whereas “AE is any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment.”6 When the suspected ADR was identified, it was assessed using two instrumental tools: WHO Causality Scale7 and Hartwig and Siegel Severity Scale.8 The drug suspected of causing ADR was then mapped to FDA-approved or off-label use data once it was identified, assessed, and documented.9
STATISTICAL EVALUATION
Mann–Whitney U test and descriptive statistics were used to summarize and compare the patient population (gender and age) disease severity with and without ADRs. Overall utilization of each off-label drug was also reported in descriptive statistics as a percentage of off-label prescription to the total prescription of each drug. Spearman’s rank correlation was used for assessment of the linear link between the total number of medications received and the occurrence an ADR. A p-value of ≤0.05 was noted as significant. STATA Software, version 13.0, was used to determine statistical analyses.
RESULTS
An analysis of 360 patients yielding 3574 medications was performed. Of these medications, 1,453 (41%) were off-label indications and 65 (1.81%) were off-label dose. On the evaluation of off-label indication use, 1,279 (88%) were evidence-based and 174 (12%) were low/no EBMs; 59 (91%) were evidence-based and 6 (9%) were low/no EBMs for off-label dose. No off-label population and route of administration was observed in the study. Three hundred sixty patients received at least one off-label medication during their ICU stay.
Off-label drug use according to the class of medication revealed 96% use of gastrointestinal agents for off-label indications where all were evidence-based, among which pantoprazole (97.9%) and ondansetron (95.42%) were the most commonly (Table 1). Followed by the antiasthmatic drug, salbutamol available in combination with ipratropium bromide was 89.7% prescribed for off-label indications of which 82.7% were evidence-based and 17.3% were low/no evidence-based. Least prescribing for off-label indications was seen in antihyperlipidemia (11.57%) and diuretic (11.2%) class of medications (Fig. 1).

Fig. 1: Off-label drugs most frequently implicated in causing ADRs
| Class of medication | Off-label medications | |||||
|---|---|---|---|---|---|---|
| Total | Evidence n (%) | Indications | Low/no evidence n (%) | Indications | ||
| Gastrointestinal | Pantoprazole | 273 | 273 (100) | Drug-induced gastrointestinal disturbances, prophylaxis | – | – |
| Ondansetron | 292 | 292 (100) | Drug-induced nausea and vomiting, prophylaxis | – | – | |
| Racecadotril | 11 | 11 (100) | Diarrhea, acute | – | – | |
| Antiasthmatic drug | Salbutamol | 52 | 43 (82.7) | Chronic obstructive pulmonary disease | 9 (17.3) | Bronchospasm–mechanical ventilation |
| Opioid | Fentanyl | 34 | 34 (100) | Analgesia for mechanically ventilated patients, procedural sedation–analgesia | – | – |
| Vasodilator | Nicorandil | 18 | 18 (100) | Congestive heart failure, ischemic heart disease, stable angina, unstable angina, variant angina | – | – |
| Steroid | Budesonide | 28 | 28 (100) | Chronic obstructive pulmonary disease, asthma–pregnancy | – | – |
| Antiarrhythmic | Digoxin | 12 | 12 (100) | Cor pulmonale–myocardial infection, supraventricular arrhythmia, supraventricular tachycardia, recurrent; prophylaxis | – | – |
| Cardiovascular | Calcium gluconate | 10 | – | – | 10 (100) | Hyperkalemia |
| Anti-infective | Oseltamivir | 10 | 10 (100) | Community-acquired pneumonia | – | – |
| Meropenem | 10 | 10 (100) | Hospital-acquired pneumonia, community-acquired pneumonia | – | – | |
| Azithromycin | 18 | 18 (100) | Community-acquired pneumonia, lower respiratory tract infection, prophylaxis | – | – | |
| Metronidazole | 16 | 11 (68.7) | Operative procedure on head—postoperative infection, prophylaxis | 5 (31.3) | Diarrhea, persistent | |
| Teicoplanin | 5 | – | – | 5 (100) | Community-acquired pneumonia, hosptal-acquired pneumonia | |
| Vancomycin | 2 | 2 (100) | Bacterial meningitis, nosocomial pneumonia | – | – | |
| Antiepileptic | Levetiracetam | 21 | 21 (100) | Partial seizure, monotherapy in newly diagnosed or untreated epilepsy, seizure. Neuroprotective, prophylaxis seizure | – | – |
| Antifibrinolytic | Tranexamic acid | 5 | 4 (80) | Postpartum hemorrhage; prophylaxis, gastrointestinal hemorrhage | 1 (20) | Epistaxis |
| Anticoagulant | Aspirin | 13 | 13 (100) | Angina pectoris, NSTEMI, venous thromboembolism, recurrent, prophylaxis, myocardial infection prophylaxis | – | – |
| Heparin | 41 | 41 (100) | Acute STEMI, angina pectoris, deep vein thrombosis, acute coronary syndrome, cerebrovascular accident | – | – | |
| Enoxaparin | 18 | 13 (72.2) | NSTEMI, cerebrovascular accident | 5 (27.8) | Arterial thrombosis | |
| Warfarin | 3 | – | – | 3 (100) | Portal vein thrombosis, cerebral venous sinus thrombosis | |
| Antihyper lipidemic | Atorvastatin | 11 | 11 (100) | Coronary arteriosclerosis, cerebrovascular accident, prophylaxis, chronic heart failure | – | – |
| Diuretic | Furosemide | 16 | 16 (100) | Acute renal failure | – | – |
There were 383 ADRs identified, out of which 139 (36.2%) were related to the off-label drug use, of these 122 (87.8%) were due to evidence off-label medication and 17 (12.2%) were due to low/no evidence off-label medication. Total number of drugs received and ADRs identified were observed to have a moderate linear association (p < 0.001); a similar association was seen with off-label drugs (p < 0.001) including evidence (p < 0.001) and low/no evidence off-label drugs (p < 0.001).
Patient characteristics, such as age, gender, acute physiology and chronic health evaluation (APACHE) II score, and the length of ICU stay, were compared for the occurrence of ADRs. A statistically significant difference was obtained for the occurrence of ADRs and patient’s length of stay (p = 0.008) (Table 2).
| Characteristic | Total | ADR | No ADR |
|---|---|---|---|
| Age, median (IQR) | 58 (43–66) | 58 (45–69) | 57 (39–65) |
| Sex | |||
| Male | 221 | 96 | 125 |
| Female | 139 | 66 | 73 |
| ICU length of stay,median (IQR) | 3 (2–4) | 3 (2–4) | 2 (1–3) |
| APACHE II score, mean (SD) | 15.7 (8.9) | 21.3 (7.4) | 12.5 (6.4) |
On review of the type of ADRs, constipation was found to be the most common ADR due to off-label drug ondansetron followed by amiodarone, meropenem and fentanyl. Suspected drug to cause fever was ondansetron followed by vancomycin and clobazam (Table 3).
| ADR | Drug implicating |
|---|---|
| Constipation | Ondansetron Amiodarone Meropenem Fentanyl |
| Fever | Clobazam Vancomycin Ondansetron |
| Hypokalemia | Fentanyl Digoxin |
| Hypotension | Diltiazem Nicorandil Furosemide |
| Thrombocytopenia | Linezolid Ceftriaxone |
| Headache | Ondansetron Nicorandil |
| Vomiting | Azithromycin Levetiracetam |
| Hematuria | Heparin |
Moreover, we found that the drugs most frequently implicated in causing ADRs due to its off-label indication with evidence are ondansetron followed by heparin and fentanyl and ADRs due to drugs with low/no evidence indication was mostly observed in (Fig. 2).
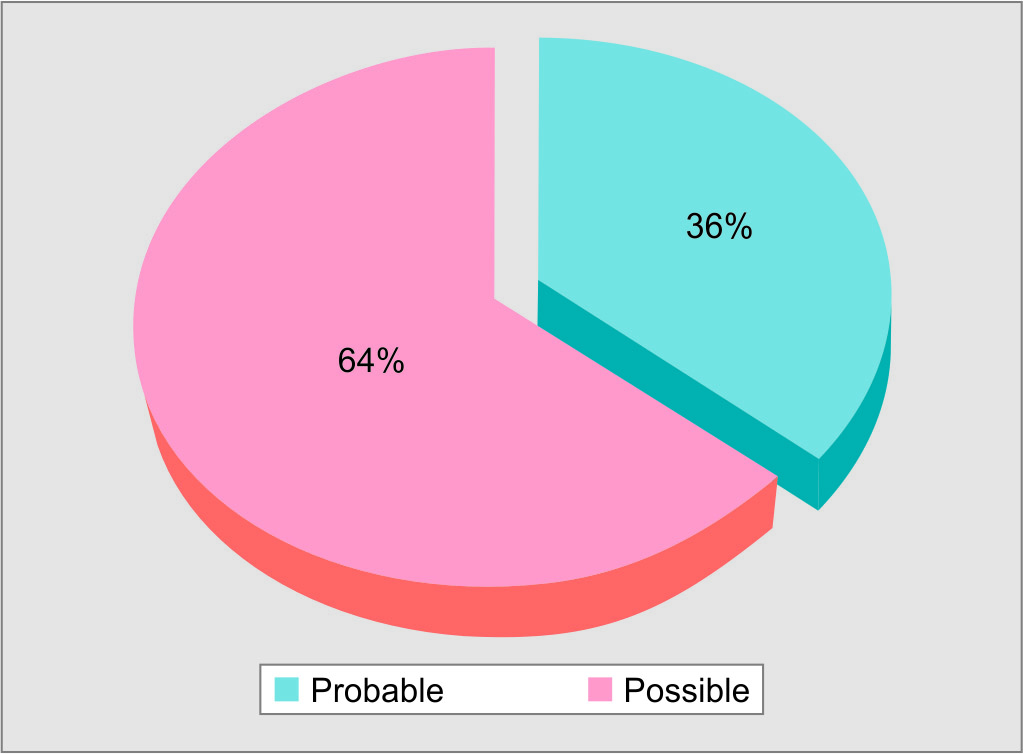
Fig. 2: Causality assessment of ADRs (defined as per WHO Causality Assessment Scale)
Causality distribution of 139 total off-label ADRs for probable and possible causality (defined as per WHO Causality Assessment Scale) was 50 (36%) and 89 (64%) respectively (Fig. 3).
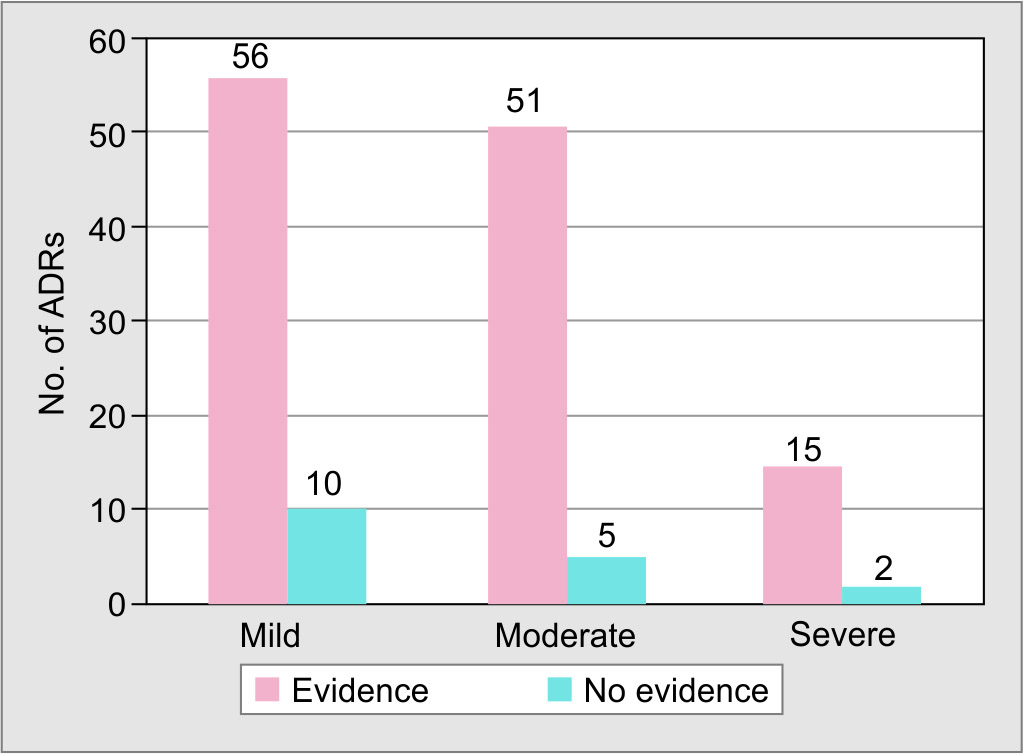
Fig. 3: Severity assessment of ADRs (defined as per Hartwig and Siegel Severity Scale)
ADRs found in the study were non-fatal in nature and no death or permanent harm due to an ADR was observed. The severity of the ADRs was classified into “mild”, “moderate” and “severe” ADRs as per Hartwig and Siegel severity scale (Fig. 3). Of 139 off-label ADRs identified in the study, 66 (47.4%) ADRs were mild in nature. It was identified out of 17 total severe ADRs caused by off-label drugs, 7 (41.17%) were due to cardiovascular abnormalities, 5 (29.4%) haematological dysfunction and 2 (11.8%) acute renal failure.
DISCUSSION
On the evaluation of medication use in 360 patients, it was observed that the prevalence of off-label was 41% of the medication prescribed. This rate is greater than 36% of off-label use reported by Ishaq Lat et al.4 and comparative to 43% of off-label use observed by Smithburger et al.9 In the present study, about 88% of off-label indication use were evidence-based and 12% were low/no evidence-based, while in Smithburger et al.’s study,9 about 51.7% were observed as low/no evidence-based. Different study designs and assessment methods could explain this disparity. Many of the off-label medications used were either a logical extension of FDA-approved indication or generalized from the approved indication for other drugs of the same class.4 For example, the FDA-approved use of an intravenous proton pump inhibitor like pantoprazole is for erosive esophagitis, but it can be effective for treating a stress-related mucosal disease which is an off-label use.4,10
Off-label drug use does not always imply that it is not supported by evidence; however, lack of regulation and research raises concern for medication safety.9 “Although the intrinsic nature of the drug to cause ADRs is the same for every drug, it may be modified by several factors including the off-label disease condition.” In the present study, the results indicate that the occurrence of ADR associated with off-label medication is 36.2% of which 87.8% was due to evidence-based and 12.2% was due to low/no evidence. Smithburger et al.9 concluded that there were no significant differences in the frequency and severity of ADRs between FDA-approved drugs (56%) and off-label medication use (44%). Variation in results between the two studies might be because they have considered only those ADRs attributed either from the off-label use or FDA-approved use.9 In comparison to other studies conducted in the critical care units of an Indian tertiary care hospital, the current study found a higher incidence of ADRs.11,12 The frequency of ADRs differs in studies due to different methods of ADR detection. We also found that there is a moderate correlation between the number of total medications received and the number of ADRs patient developed.9 This finding is in line with a previously identified drug-related risk factor related to the development of ADR in patients taking high-alert medications (heparin and sedatives) administered to critically ill patients.9,13
The present study detected distinct observation in the prevalence of gastrointestinal and immunologic drugs prescribing as evidence and low/no evidence, but prescribing pattern of broncho respiratory class of drugs was comparable with Ishaq Lat et al.4 Also present study, antiasthmatic and cardiovascular drugs, such as a vasodilator and antiarrhythmic drugs, among the most frequently prescribed as off-label drugs, which is similar to the study by Golocorbin Kon et al.3 that included cardiovascular drugs, anticonvulsants and anti-asthmatic drugs. Fentanyl, a powerful, fast-acting opiate analgesic indicated for severe pain in patients with carcinoma, is the best example of off-label prescribing of a drug.3 The data available from Golocorbin Kon et al, 80% of fentanyl was prescribed as off-label.3
The literature reveals that “most drug reactions tend to occur in the first 5 days on a drug.”14 In the present study, the occurrence of ADR is mostly observed in 1 to 6 days of patient’s admission, which was consistent with the other studies.11,15 This may be due to the fact that the patients during the initial stay in the ICU are unstable and have a serious condition that requires frequent high-risk decision-making usually with insufficient data.15 Also, such patients receive multiple drug therapies and are exposed to high-alert medications such as heparin and insulin.9,13,16 Most of the time patients are treated symptomatically, which may temporarily resolve the symptoms but do not treat the underlying cause, which increases the risk of ADR in immunocompromised patients. Only patients with less severe conditions stay in the intensive care for a short time, while patients who died in the first few days had no adverse reactions. However, patients who stay for an extended period of time are more likely to be more critically ill and experience adverse reactions.17 According to the findings of this study, the median length of stay in the patients with ADR was higher (p = 0.008).
In the present study, ADRs attributed to off-label use was 36.2%. The five most common ADRs seen were constipation, fever, hypokalemia, hypotension, and thrombocytopenia. Constipation was due to ondansetron followed by amiodarone, meropenem and fentanyl. Patients developed a fever after three doses of ondansetron injection. The commonly offending drugs were ondansetron, heparin, ceftriaxone, fentanyl, and furosemide. Smithburger et al.9 have considered ADRs without differentiating the drugs into their FDA-approved or off-label association, and most ADRs were caused by corticosteroids and opioids. There is an inconsistency between the results of both studies, either due to the fact that the studies have been conducted in two different countries with different patient demographics and hospital setup or because the method of detection of ADRs was different in both the studies. They only considered those ADRs that could be classified into two categories due to off-label or FDA-approved use, whereas we looked at all possible ADRs associated with off-label medications.9 They adopted an active surveillance strategy in which a clinical pharmacist evaluated patients for ADRs, whereas we have identified and analyzed ADRs during the ward rounds and by reviewing the medical charts.
Most of the ADRs identified in the present study showed “possible” (58%) causal relationship with the suspected drug as there were other contributing factors, such as concomitant drugs and comorbid conditions attributing to the development of ADRs. The majority of ADRs observed in the present study were mild in nature (47.4%), while severe ADRs were less in number (12.2%); this result is in accordance with the other studies.9,16 Severe ADRs, such as hematological dysfunction, cardiovascular abnormalities, acute renal failure, was noticed in the present study, which is the same as those of Joshua et al.11 The severe ADRs which caused an increase in stay, requiring intensive care were either treated symptomatically or the drug causing ADR was withdrawn. However, the treatment of ADRs is outside the scope of this study.
All studies conducted showed that the prevalence of off-label drug use is significant, which is also observed in the present study. Drugs are labeled for an indication when the FDA carefully reviews its safety and efficacy and is usually considered by physicians for prescribing. When the physician surpasses the boundaries of prescribing labeled indication, they mostly rely on their practice experience in deciding how to use an approved agent. In some instances off-label prescribing is clearly in the patient’s best interest in a particular clinical situations.18 Off-label use is frequently justified by published scientific evidence that supports it or in data-deficient clinical situations where the theoretical benefit exceeds the potential risk.9
Off-label prescribing decisions are frequently justified by clinical and research-based information based on EBM.3 “The concept of EBM encourages some doctors to administer off-label drugs if such administration is appropriate in given circumstances.”3 But many clinicians do not have the time or motivation to review the evidence for those off-label indications in order to seek a balanced risk–benefit assessment to support the rational use of the drug, because the FDA does not control the drug-prescribing practice.18 Perhaps the high prevalence of off-label drug use is due to healthcare providers’ lack of understanding of FDA indications, with the common assumption that FDA approval for off-label indications is with low or no proof of benefit. In addition, physicians are not required by law to follow the approved labeled indications, and the FDA even acknowledges that in some cases, off-label use of drugs may constitute good medical practice.18 There are many examples of appropriate off-label medication use. In some cases, use of off-label drugs may be the standard of care. For example, digoxin is approved for rate control in atrial fibrillation, but metoprolol use is off-label. However, evidence-based clinical guidelines recommend metoprolol over digoxin as the first-line therapy for rate control.18
The restricted study duration of 6 months may have underestimated the prevalence of off-label use as well as the occurrence of ADRs, thus ongoing monitoring of off-label use would help in raising awareness of such practice. All the possible ADRs, with having FDA-approved drug as one of the causal medications, were included in the study, which prevented identifying the incidence of ADRs related only to off-label drug use. Patient medication chart review along with clinical review was conducted on a routine basis to identify ADRs. However, some ADRs that were not reported and documented in medication charts have not been detected due to restricted entry into the ICU and nonmonitoring of the patient’s bedside for full duration of that day. This could be the reason for the underestimation of ADRs in ICU. Finally, incident reporting was subjected to institutional and unit culture as well as laboratory reports ordered by a physician with treatment and diagnosis point of view irrespective of those specific for ADRs identified.
Patients admitted to the ICU were critically ill either with already-diagnosed conditions or comorbidity or with severe illnesses, thus making it difficult to identify ADRs. Also, there were cases wherein patient had received medications in other hospitals and departments before admission to the ICU, which were not included in our study that could have led to misinterpreting the occurrence of an ADR with ICU treatment. Our findings may not be generalized to surgical and neurology ICUs as well as nonteaching hospitals where the severity of the disease or the type of patient can be significantly different.
CONCLUSION
Widespread presence of off-label use was observed in medical ICU. Although incidence of ADRs was similar to the FDA-approved use, ongoing monitoring of such practice is needed.
ORCID
Asawari Raut https://orcid.org/0000-0002-3262-1755
Kavita Krishna https://orcid.org/0000-0003-0632-6231
Utkarsha Adake https://orcid.org/0000-0001-6902-2441
Apurva A Sharma https://orcid.org/0000-0001-8439-071X
Anitta Thomas https://orcid.org/0000-0003-0378-6048
Jignesh Shah https://orcid.org/0000-0002-8812-8791
REFERENCES
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc 2012;87(10):982–990. DOI: 10.1016/j.mayocp.2012.04.017.
2. Gazarian M, Kelly M, McPhee JR, Graudins LV, Ward RL, Campbell TJ. Off-label use of medicines: consensus recommendations for evaluating the appropriateness. Med J Aust 2006;185(10):544–548. DOI: 10.5694/j.1326-5377.2006.tb00689.x.
3. Goločorbin Kon S, Iliković I, Mikov M. Reasons for and frequency of off - label drug use. Med Pregl 2015;68(1-2):35–40. DOI: 10.2298/mpns1502035g.
4. Lat I, Micek S, Janzen J, Cohen H, Olsen K, Haas C. Off-label medication use in adult critical care patients. J Crit Care 2011;26(1):89–94. DOI: 10.1016/j.jcrc.2010.06.012.
5. Micromedexsolutions.com Recommendation. Evidence and efficacy ratings. In: DRUGDEX® system. Greenwood Village: Thomson Micromedex. Available at: http://www.micromedexsolutions.com
6. ich.org. Available at: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2F/Step4/E2F_Step_4.pdf (accessed 29 June 2019).
7. who.int. The use of the WHO–UMC system for standardised case causality assessment. Available at: https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf (accessed 4 Mar 2019).
8. Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992;49:2229–2231. Available at: https://pubmed.ncbi.nlm.nih.gov/1524068/
9. Smithburger PL, Buckley MS, Culver MA, Sokol S, Lat I, Handler SM, et al. A multicentre evaluation of off-label medication use and associated adverse drug reactions in adult medical ICUs. Crit Care Med 2015;43(8):1612–1621. DOI: 10.1097/CCM.0000000000001022.
10. Spirt MJ. Stress-related mucosal disease: risk factors and prophylactic therapy. Clin Ther 2004;26(2):197–213. DOI: 10.1016/s0149-2918(04)90019-7.
11. Joshua L, Devi P, Guido S. Adverse drug reactions in intensive care unit of a tertiary care hospital. Pharmacoepidemiol Drug Saf 2009;18(7):639–45. DOI: 10.1002/pds.1761.
12. Saravanan SS, Kavitha P, Ponnuswamy TK. Patterns of adverse drug reactions in the intensive care unit of an Indian tertiary care hospital. Int J Pharm Biol Arch 2014;5(3):64–68. DOI: 10.18203/2319-2003.ijbcp20192569.
13. Kane-Gill SL, Jacobi J, Rothschild JM. Adverse drug events in intensive care units: risk factors, impact, and the role of team care. Crit Care Med 2010;38(6 Suppl):S83–S89. DOI: 10.1097/CCM.0b013e3181dd8364.
14. Strom BL, Schinnar R. Hospital pharmacoepidemiology. 4th ed. West Sussex: John & Wiley Sons Ltd., 2005, pp. 539–553.
15. Ohta Y, Sakuma M, Koike K, Bates DW, Morimoto T. Influence of adverse drug events on morbidity and mortality in intensive care unit: the JADE study. Int J Qual Health Care 2014;26(6):573–578. DOI: 10.1093/intqhc/mzu081.
16. Seynaeve S, Verbrugghe W, Claes B, Vandenplas D, Reyntiens D, Jorens PG. Adverse drug events in intensive care unit: a cross-sectional study of prevalence and risk factors. Am J Crit Care 2011; 20(6):e131–e140. DOI: 10.4037/ajcc2011818.
17. Roque KE, Tonini T, Melo EC. Adverse events in the intensive care unit: impact on mortality and length of stay in a prospective study. Cad Saude Publica 2016;32(10):e00081815. doi: 10.1590/0102-311X00081815.
18. Good CB, Gellad WF. Off-label drug use and adverse drug events: turning up the heat on off-label prescribing. JAMA Intern Med 2016;176(1):63–64. DOI: 10.1001/jamainternmed.2015.6068.
________________________
© The Author(s). 2021 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.