PEDIATRIC CRITICAL CARE |
https://doi.org/10.5005/jp-journals-10071-23954 |
Practices of Initiation of Vasoactive Drugs in Relation to Resuscitation Fluids in Children with Septic Shock: A Prospective Observational Study
1Department of Pediatrics, ESIC Hospital and Medical College, Faridabad, Haryana, India
2,3Division of Pediatric Critical Care, Department of Pediatrics, Lady Hardinge Medical College and Kalawati Saran Children’s Hospital, Delhi, India
Corresponding Author: Shalu Gupta, Division of Pediatric Critical Care, Department of Pediatrics, Lady Hardinge Medical College and Kalawati Saran Children’s Hospital, Delhi, India, Phone: +91-9779577087, e-mail: drshalugupta@yahoo.co.in
How to cite this article: Karanvir, Gupta S, Kumar V. Practices of Initiation of Vasoactive Drugs in Relation to Resuscitation Fluids in Children with Septic Shock: A Prospective Observational Study. Indian J Crit Care Med 2021;25(8):928–933.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Background: The role of vasoactive medications in septic shock is well-defined, but the appropriate time of initiation of these medications in reference to fluid boluses is not clear. We planned to study prospectively the practices and outcome of initiation of vasoactive infusions with respect to resuscitation fluids boluses in pediatric septic shock.
Patients and methods: Children aged 1 month to 18 years diagnosed with septic shock were enrolled to receive fluid resuscitation boluses along with vasoactive drugs. The primary outcome was to look at various practices of the initiation of vasoactive infusions; accordingly, patients were categorized into three groups: N1 received vasoactive infusions after completion of the first bolus (20 mL/kg), N2 after the second (40 mL/kg), and N3 after the third fluid (60 mL/kg) bolus. Secondary outcomes were to compare the time taken, amount of fluid required to achieve hemodynamic stability, total fluid required, and complications in the first 24 hours of treatment and mortality.
Results: Hundred children were enrolled and grouped into N1, N2, and N3 with 46, 10, and 44 patients, respectively. The volume of fluid required to achieve the resolution of shock in N1 (40 ± 10 mL/kg) was significantly less than in N2 (70 ± 10 mL/kg) and N3 (70 ± 20 mL/kg); p = 0.02. The time taken to achieve hemodynamic stability was significantly less in N1 (115 ± 45 minutes) than in N2 (196 ± 32 minutes) and N3 (212 ± 44 minutes); p = 0.02. The volume of intravenous fluid required in the first 24 hours (p = 0.02) and complications were lower in the N1 group (p = 0.04). No statistical difference in mortality was seen.
Conclusion: Early initiation of vasoactive infusions (after the first bolus) resulted in less total fluid volume, lesser time to achieve hemodynamic stability, less fluid boluses, less length of stay in the pediatric intensive care unit, and lesser complications in the first 24 hours.
Keywords: Fluid overload, pediatric intensive care unit, Resuscitation fluid, Sepsis, Septic shock, Vasoactive drugs.
Highlight: Early initiation of vasoactive infusions—after completion of the first fluid bolus resulted in less need for further fluid boluses, lesser time for shock resolution, lesser fluid overload, and less PICU stay—in pediatric septic shock.
INTRODUCTION
Shock is a state of mismatched circulation that creates a disparity between tissue oxygen supply and oxygen demand, resulting in altered cellular and subcellular metabolism and energy production.1 Globally, the overall pediatric septic incidence amounts to 48 sepsis and 22 severe septic cases in children per 100,000 person-years, with mortality ranging from 11 to 19%.2
The guidelines recommend fluid resuscitation with boluses of 20 mL/kg to achieve an adequate blood pressure as well as optimal cardiac output parameters, which include heart rate, improvement in peripheral pulse volume, capillary refill time, conscious level, peripheral skin temperature, and urine output. Mostly this resuscitation volume is in the range of 40 to 60 mL/kg but even boluses up to 200 mL/kg of resuscitation volume have been described in the literature.3 These guidelines, however, were based on expert opinion and lacked adequately controlled pediatric evidence. However, the concept of fluid resuscitation underwent a paradigm shift. A large pediatric clinical trial highlighted that fluid boluses caused a significant increase in mortality in children diagnosed with severe febrile illness and impaired perfusion within the first 48 hours of treatment.4 This was further reiterated that fluid boluses can cause respiratory and neurological dysfunction in the resuscitation of febrile children.5 Pulmonary edema is one of the serious complications of aggressive fluid correction, leading to an increased need for mechanical ventilation.
To maintain optimal organ perfusion, vasoactive medications are indicated to maintain the mean arterial pressure, both during and following fluid resuscitation.6,7 The role of vasoactive infusions in septic shock is well-defined, but the appropriate time of initiation of these in reference to fluid boluses is not clear. Few adult studies have found that there is an increased risk of organ failures as well as decreased survival in septic shock patients with delayed vasoactive initiation,8 whereas in another study early administration of vasoactive medications in septic shock patients led to the attainment of adequate perfusion pressure.9 Although, in another retrospective study, no difference was observed in the length of ICU stay or organ dysfunction with early or late vasoactive medication administration.10 Similarly in children, early use of vasoactive medications had no difference in outcomes seen in relation to early resolution of shock and mortality.11
These lacunae in the knowledge of appropriate timing of initiation of vasoactive medication in relation to fluid boluses in the first hour of resuscitation generated the hypothesis, that prolonged periods of uncorrected hypotension with delayed initiation of vasoactive medications would ultimately lead to higher mortality. Hence we planned this study to know the practices of initiation of vasoactive medications with respect to fluid boluses during resuscitation and its outcome in pediatric septic shock.
METHODS
Participants and Setting
This study was a prospective observational study done in children aged 1 to 18 years admitted in the emergency and pediatric critical care unit of the Kalawati Saran Children’s Hospital (KSCH) and Lady Hardinge Medical College (LHMC), New Delhi, between November 2014 and April 2016 with septic shock. The KSCH is a tertiary care children’s hospital of 395 beds with 20 beds dedicated to the pediatric intensive care unit (PICU) admitting on an average of 800 patients annually. This study was approved by the institutional ethical committee. Septic shock was defined as the presence of signs of sepsis with cardiovascular dysfunction. Cardiovascular dysfunction was defined as the presence of either hypotension [systolic blood pressure (SBP) < 5th percentile or SBP < 70 mm Hg in infants or SBP (70+ age in years × 2 ) after 1 year of age] or clinical signs of hypoperfusion—namely decreased pulse volume, capillary refill time (CRT) >3 seconds, and mottled or cold extremities.12 Exclusion criteria included any child who received prior intravenous fluid boluses before admission, severe acute malnutrition, severe anemia, and diabetic ketoacidosis. Before enrollment in the study, parents were informed about the study and written consent was taken from them.
Groups
Since this was a pilot study, a sample size of 100 patients was taken. The amount of fluid received and the time of initiation of vasoactive infusions were recorded for all the cases. These 100 patients were accordingly categorized into three groups based on the initiation of vasoactive medications with respect to resuscitation fluid boluses: N1 group received vasoactive medications after completion of the first fluid bolus (20 mL/kg), N2 group after the second fluid bolus (40 mL/kg), and N3 after the third fluid bolus (60 mL/kg).
Outcome Measures
The primary outcome was to observe the number of patients receiving vasoactive medications in relation to fluid boluses. The secondary outcome was to analyze for the volume of fluid boluses administered, time taken to achieve shock resolution, total amount of fluid received in the first 24 hours of management, complications, duration of vasoactive medications, and mortality among these three groups.
Methodology
A standard protocol was followed for managing children admitted with septic shock.3 A strict compliance regarding the adherence to protocol was ensured with each enrolled participant. The decision to start vasoactive support was taken on a case-to-case basis by the treating physician on duty, based on clinical assessment. All children at the onset received normal saline boluses of 20 mL/kg to a maximum of 60 mL/kg with meticulous monitoring of vital signs and fluid overload features after each bolus. Fluid overload was diagnosed clinically based on the presence of any one of the following: new or worsening hepatomegaly, appearance of gallop rhythm or basal creps/rales, worsening of respiratory status, i.e., an increase in respiration rate from baseline (≥5), or radiological evidence of pulmonary edema on chest X-ray (the presence of perihilar haze, or septal lines, or thickening of interlobar fissure, or batwing opacification).
After the first hour of resuscitation, further need for fluid bolus was guided by the inferior vena cava (IVC) collapsibility/distensibility indices and cardiac echocardiography along with clinical features of hypoperfusion. An age-based IVC nomograms were used for guiding further fluid therapy.13 Titration of vasoactive infusions to optimize tissue perfusion was done as per the standard protocol.3 Shock resolution were defined as the achievement of normal pulse rate for age, improvement of peripheral pulse volume, capillary refill time <3 seconds, systolic blood pressure >5th centile, and urine output more than 1 mL/kg/hour. In case the child persisted to have hemodynamic instability with failure to achieve the therapeutic end point of shock, or developed fluid overload or respiratory failure, the child was intubated.7 Further a uniform protocol was followed for the optimization of ventilation.
A normal saline fluid bolus of 20 mL/kg was administered over 15 to 20 minutes.7 A stop clock was used by the treating team during the first hour for keeping the time as starting of vasoactive infusions. To ensure the compliance of protocol during the study, training of residents was done prior to the study enrolment through simulated case scenarios.
Measurements and Data Recording
All demographic characteristics and baseline hemodynamic parameters were recorded in a predesigned performa. Clinical details and investigations, like blood samples for electrolytes, hemogram, and blood gas pH, were recorded prior to a fluid bolus. Therapeutic decisions about the addition of fluid bolus and its amount were decided by the treating unit as per the protocol.3 The total duration of vasoactive infusions used and signs of fluid overload complication, like facial puffiness, hepatomegaly, and pulmonary edema, were recorded in these patients. The Pediatric Risk of Mortality (PRISM) III score was used to assess the severity of illness at 12 hours of admission.
Statistical Methods
Continuous variables were expressed as mean ± standard deviation (SD) and were compared using the Student t-test. Categorical variables were analyzed using the chi-square test or Fischer’s exact test as appropriate. Means among three groups were compared by analysis of variance (ANOVA) test. A p value (two-tailed) less than 0.05 was considered significant. SPSS 19.0 (SPSS, Chicago, Illinois) was used for data analyses.
RESULTS
A total of 168 patients were screened, out of which 68 patients were excluded and 100 cases were enrolled (Flowchart 1). There was no significant difference in the demographic characteristics, lab values, and hemodynamic parameters among the three groups on admission in the pediatric emergency (Table 1). Out of the 100 patients, 56% were females and 44% were males.

Flowchart 1: Study flowchart
| Characteristics | N1 (n = 46) | N2 (n = 10) | N3 (n = 44) | p value |
|---|---|---|---|---|
| Age years (mean ± SD) | 7.8 ± 1.9 | 8.1 ± 1.2 | 7.6 ± 2.1 | 0.32 |
| Duration of illness (days) | 4.5 ± 2 | 5.4 ± 1.7 | 5.2 ± 1.9 | 0.23 |
| Weight (kg) | 23.12 ± 7.14 | 26.5 ± 6.3 | 23.8 ± 5.7 | 0.60 |
| Height (cm) | 122.9 ± 21.7 | 126.9 ± 18.8 | 118.5 ± 24.9 | 0.48 |
| Glasgow coma scale (GCS) | 11 ± 2 | 12 ± 2 | 12 ± 2 | 0.71 |
| PRISM—3 | 12 ± 4 | 9 ± 2 | 10 ± 4 | 0.60 |
| Focus of infection | ||||
| Dengue shock syndrome | 20 | 7 | 22 | |
| Skin and soft tissue | 14 | 2 | 9 | |
| Lungs | 7 | 1 | 6 | |
| GIT | 0 | 0 | 3 | |
| Enteric fever | 2 | 0 | 0 | |
| Meningitis | 2 | 0 | 2 | |
| Severe malaria | 1 | 0 | 2 | |
| Hemodynamic parameters | ||||
| Heart rate (beats/minute) | 130.8 ± 22 | 132.4 ± 17.7 | 138 ± 19.2 | 0.69 |
| Respiration rate (breaths/minute) | 33.1 ± 11.1 | 28.4 ± 5.7 | 32.3 ± 13.3 | 0.52 |
| Systolic BP (mm Hg) | 56.5 ± 29.1 | 66.6 ± 12.7 | 62.4 ± 28.7 | 0.56 |
| Diastolic BP (mm Hg) | 39.5 ± 9.2 | 46.7 ± 14.6 | 44.7 ± 8.6 | 0.64 |
| Mean arterial pressure (mm Hg) | 44.5 ± 10.1 | 50.6 ± 11.6 | 52.3 ± 16.3 | 0.31 |
| Laboratory variables | ||||
| Hemoglobin (gm/dL) | 11.1 ± 2.2 | 11.0 ± 1.2 | 11.4 ± 2.1 | 0.72 |
| Total leukocyte count (×109/L) | 14.3 ± 2.4 | 9.8 ± 1.2 | 9.9 ± 2.1 | 0.08 |
| Platelet count (×109/L) | 121 ± 11.6 | 148.8 ± 37 | 106 ± 29.6 | 0.12 |
| Blood sugar (mg/dL) | 97.4 ± 20.6 | 101 ± 22.3 | 99.8 ± 21.6 | 0.78 |
| Blood urea (mg/dL) | 34.1 ± 18.3 | 29.5 ± 9.84 | 34.2 ± 13.6 | 0.85 |
| Serum creatinine ( mg/dL) | 0.5 ± 0.2 | 0.4 ± 0.2 | 0.5 ± 0.1 | 0.59 |
| Serum albumin (gm/dL) | 2.2 ± 0.6 | 2.4 ± 0.3 | 2.6 ± 0.6 | 0.37 |
| Total serum bilirubin (mg/dL) | 0.9 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.2 | 0.45 |
| Serum lactate (mmol/L) | 2.4 ± 1.4 | 2.2 ± 0.9 | 2.5 ± 1.6 | 0.81 |
| Blood pH | 7.27 ± 0.08 | 7.32 ± 0.07 | 7.29 ± 0.08 | 0.22 |
Primary Outcome (Table 2)
Forty-six patients (N1) received vasoactive medications after completion of the first resuscitation fluid bolus (20 mL/kg), 10 (N2) received after completion of the second bolus (40 mL/kg), and 44 (N3) received after completion of the third fluid bolus (60 mL/kg).
Secondary Outcome
The volume of fluid required for shock resolution in the N1 group was 40 ± 10 mL/kg, which was significantly less than in the N2 group 70 ± 10 mL/kg and N3 group 70 ± 20 mL/kg (p = 0.02) (Table 2). The time taken to achieve resolution of shock was significantly less in N1 (115 ± 45 minutes) than in N2 (196 ± 32 minutes) and N3 (212 ± 44 minutes) (p = 0.02). Number of patients developing features of fluid overload, like facial puffiness, hepatomegaly, and pulmonary edema, were lower in N1 (15 cases, 32%) than in the N2 (4 cases, 40%) and N3 (21 cases, 47%) (p = 0.04) groups. The volume of fluid received in the first 24 hours of treatment in N1 (122 ± 22 mL/kg) was significantly lower than in the N2 (151 ± 31 mL/kg) and N3 (145 ± 24 mL/kg) groups (p = 0.02). The duration of vasoactive medications used in the N1, N2, and N3 group was 33 ± 4.6 hours, 34 ± 2.3 hours, and 38 ± 1.8 hours, respectively, with no significant difference (p = 0.45). Vasoactive medications used in these three groups were dopamine or/and epinephrine or/and norepinephrine or/and dobutamine. The maximum vasoactive-inotropic score (VIS) in N1, N2, and N3 was 28.2 ± 1.9, 34 ± 2.1, and 31.3 ± 2.3 (p = 0.19), respectively. The number of patients who required mechanical ventilation was lower in N1 (12 cases, 27%) as compared to N2 (4 cases, 40%) and N3 (18 cases, 38%) (p = 0.03). The duration of mechanical ventilation in N1, N2, and N3 groups was 41 ± 3.7, 46 ± 1.7, and 43 ± 2.6 hours, respectively, with no statistical difference (p = 0.37). Mortality among N1, N2, and N3 was 17, 30, and 27%, respectively, with no statistically significant difference (p = 0.25). The length of PICU stay in N1 was 5 ± 1 days, which was statistically lower than the other two groups, i.e., N2, 6 ± 2 days and N3, 7 ± 2 days (p = 0.03). The first 24 hours trends in heart rate and mean blood pressure are shown in Figures 1 and 2, respectively.

Fig. 1: Heart rate trends during the first 24 hours
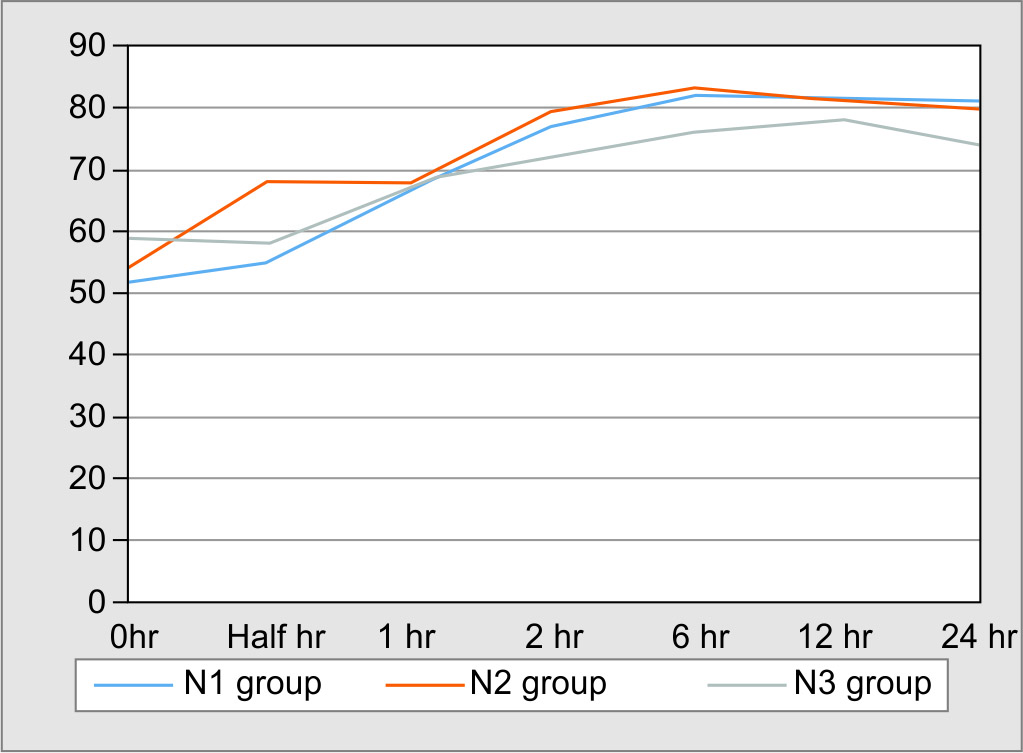
Fig. 2: Mean arterial pressure trends during the first 24 hours
| Outcome variables | N1 (n = 46) |
N2 (n = 10) |
N3 (n = 44) |
p value |
|---|---|---|---|---|
| Volume (mL/kg) of fluid for shock reversala | 40 ± 10 | 70 ± 10 | 70 ± 20 | 0.02 |
| Time (minutes) taken for shock reversala | 115 ± 45 | 196 ± 32 | 212 ± 44 | 0.02 |
| Duration of inotropes (hours)a | 33 ± 4.6 | 34 ± 2.3 | 38 ± 1.8 | 0.45 |
| Fluid overload, n (%)b | 15 (33) | 4 (40) | 21 (48) | 0.04 |
| Pulmonary edema, n (%)b | 4 (8) | 3 (30) | 10 (23) | 0.03 |
| Volume (mL/kg) of fluid given in the first 24 hoursa | 122 ± 22 | 151 ± 31 | 145 ± 24 | 0.02 |
| Maximum vasoactive-inotropic score (VIS)a | 28 ± 1.9 | 34 ± 2.1 | 31.3 ± 2.3 | 0.19 |
| Mechanical ventilation, n (%)b | 12 (27) | 4 (40) | 18 (38) | 0.03 |
| Duration of mechanical ventilation (hours)a | 41 ± 3.7 | 46 ± 1.7 | 43 ± 2.6 | 0.37 |
| Mortality, n (%)b | 8 (17) | 3 (30) | 12 (27) | 0.25 |
| Length of PICU stay (days)a | 5 ± 1 | 6 ± 2 | 7 ± 2 | 0.03 |
DISCUSSION
In pediatric septic shock, the outcome of management mainly depends upon the resuscitation with fluid boluses, early use of vasoactive medication, and timely antimicrobial administration. However, the timing of starting vasoactive drugs is not clear. In an adult study, a significant association was observed between delay in starting vasopressors of >14 hours and onset of various new organ failures, like renal, coagulation, central nervous system, respiratory system, and metabolic.8
In our study, we observed that in a large majority of patients, vasoactive drugs were initiated after completion of the first fluid bolus and the least number after completion of the second resuscitation fluid bolus. Forty-four percent of children received three boluses prior to starting the vasoactive drugs. This variability observed in initiation of vasoactive infusions at a different time in our study was possibly contributed by many factors, such as heightened awareness to fluid overload in response to fluid boluses, individual variability in the clinical spectrum of the disease, and some degree of variability in clinical assessment by the treating physician. The volume of resuscitation fluid required for the reversal of shock, time taken for achieving end points of shock, and fluid volume required in the initial 24 hours of treatment were significantly less in the first (N1) group where vasoactive medications were initiated after completion of the first fluid bolus, which highlights the point that early initiation of vasoactive drugs mitigates the need for fluid boluses and its associated complications. Our findings are in congruence with an adult study where early initiation of norepinephrine in septic shock patients increased mean arterial pressure, cardiac index, and stroke volume index irrespective of the amount of fluid given during resuscitation.9 Our findings can be explained by two mechanisms. Firstly, the early use of vasoactive drugs surpassed the sepsis-induced decreased responsiveness of the myocardium to beta-adrenergic stimulation.14 Secondly, tachyphylaxis of beta-1-adrenergic receptors leading to the downregulation of these receptors is less likely as none of the patients enrolled in our study received any prior vasoactive medication.15 However, our findings are in contrast to a previous pediatric study, where no difference was observed between the two groups with regards to the resolution of shock, mortality rate, and number of patients intubated.11 This could probably be explained because in the study group, dopamine was started after 40 mL/kg fluid bolus and in the control group, dopamine was started after completion of 60 mL/kg of fluid bolus at 1 hour.11 However, they found that the new appearance of hepatomegaly at the first 20 minutes after treatment was higher in the study group as compared to the control group. Similarly, in a retrospective adult study (n = 95), no difference was observed between the two groups (early vasopressors administration group <1.37 hours and late vasopressors administration group) with regards to the organ dysfunction or length of ICU stay.10
We found no significant difference between the mean duration of use of vasoactive medications among the three groups, which is consistent with the previous study,11 and this possibly indicates toward underlying disease pathophysiologic state. Forty-seven percent of children developed fluid overload complications in the N3 group followed by 40% in the N2 group. Thirty-two percent of children developed fluid overload complications even after the first bolus. Santhanam et al. reported a higher rate of hepatomegaly at the end of the initial 20 minutes of treatment in their study group, which received 40 mL/kg fluid bolus (52/74 vs 26/73, p = 0.001), as compared to the control group, which received 20 mL/kg fluid bolus in the same time.11 These findings of fluid overload complications especially pulmonary edema would lead to early intubation and the use of mechanical ventilation. This facility of mechanical ventilation might not be available everywhere in a resource-limited setting. Moreover, in developing country, children with underlying malnutrition and septic shock are more prone to develop fluid overload complications with aggressive fluid management.
There was no statistically significant difference in mortality among the three groups (N1, N2, and N3), though the mortality was lesser in the N1 group than in N2 and N3 groups. This finding of lesser mortality in N1 is similar to a previous retrospective adult study where the interval between diagnosis of septic shock and the administration of vasopressor agents had a modest independent correlate to the inhospital mortality and development of late organ failure.8 However, the authors highlighted that increasing mortality (OR = 1.34, 95% CI = 1.03–1.76, p = 0.048) was seen in the group where vasopressors were administered after 14 hours from the time of diagnosis of septic shock.8 On the contrary, Subramanian et al. did not observe any organ dysfunction in the study group even up to 12 hours delay in the administration of vasopressor.10 Probably there are other factors that play a role in optimizing the organ perfusion apart from the vasoactive drugs. The length of PICU stay was significantly increased with the number of fluid boluses. This could be explained due to the increased fluid-related complications, leading to their increased ICU stay. Contrary to our findings, the intubation rate in the treatment group (55%) was not significant from the control group (46.5%) with p value = 0.28; also no statistical difference was noted between these two groups for the length of stay in the hospital.11
The limitation to this study was that we did not study the interobserver variability in detecting the signs of shock, which probably could have led to bias. We did not use invasive hemodynamic monitoring, and it was an observational study. Another limitation of our study was that majority of children enrolled later turned out to be dengue shock syndrome. A septic shock-like state can be caused by various infectious organisms, and therefore, in an emergency when the child presents in shock, it is difficult to categorize the etiology of shock. Moreover, our primary objective was irrespective of the underlying pathophysiology.
The strength of the study is that it is the first pediatric study that highlights the early use of vasoactive infusions with respect to fluid boluses in pediatric septic shock.
CONCLUSION
The initiation of vasoactive medications with completion of the first bolus of 20 mL/kg resulted in less need for further fluid boluses, lesser time for shock resolution, fewer fluid overload complications, and lesser length of stay in PICU. The mortality rate among the three groups was not statistically significant, so it cannot be concluded that the mortality rate was lesser. Further randomized control trials are needed to validate this aspect of early initiation of vasoactive medications.
ACKNOWLEDGMENTS
All the nurses and residents in the pediatric emergency and pediatric critical care unit of the KSCH, New Delhi, during the study period.
Contribution: Karanvir and Virendra Kumar conceptualized and designed the study. Karanvir collected the data, analyzed, and prepared the initial draft. Shalu Gupta critically reviewed the manuscript and prepared the final draft. Virendra Kumar reviewed and gave final approval.
ORCID
Karanvir https://orcid.org/0000-0003-3851-5336
Shalu Gupta https://orcid.org/0000-0003-0494-0465
Virendra Kumar https://orcid.org/0000-0001-6752-9281
REFERENCES
1. Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med 2003;167(5):695–670. DOI: 10.1164/rccm.200207-682OC.
2. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med 2018;6(3):223–230. DOI: 10.1016/S2213-2600(18)30063-8.
3. Brierly J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, et al. Clinical practice, parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from American College of Critical Care Medicine. Crit Care Med 2009;37(2):666–688. DOI: 10.1097/CCM.0b013e31819323c6.
4. Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in Africa children with severe infection. N Engl J Med 2011;364(26):2483–2495. DOI: 10.1056/NEJMoa1101549.
5. Levin M, Cunnington AJ, Wilson C, Nadel S, Lang HJ, Ninis N, et al. Effects of saline or albumin fluid bolus in resuscitation: evidence from re-analysis of the FEAST trial. Lancet Respir Med 2019;7(7):581–593. DOI: 10.1016/S2213-2600(19)30114-6.
6. Le Doux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med 2000;28(8):2729–2732. DOI: 10.1097/00003246-200008000-00007.
7. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign guidelines committee including the pediatric subgroup: surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41(2):580–637. DOI: 10.1097/CCM.0b013e31827e83af.
8. Beck V, Chateau D, Bryson LG, Pisipati A, Zanotti, Parrillo JE, et al. Timing of inotropes initiation and mortality in septic shock. Crit Care Med 2014;18(3):R97. DOI: 10.1186/cc13868.
9. Hamzaoui O, Georger JF, Monnet X, Ksouri H, Maizel J, Richard C, et al. Early administration of nor epinephrine increases cardiac preload and cardiac output in septic patients with life-threatening hypotension. Crit Care 2010;14(4):R142. DOI: 10.1186/cc9207.
10. Subramanian S, Yilmaz M, Rehman A, Hubmayr RD, Afessa B, Gajic O. Liberal vs. conservative vasopressor use to maintain mean arterial blood pressure during resuscitation of septic shock: an observational study. Intensive Care Med 2008;34(1):157–162. DOI: 10.1007/s00134-007-0862-1.
11. Santhanam I, Sangareddi S, Venkataraman S, Kissoon N, Thiruvengadamudayan V, Kasthuri RK. A prospective randomized controlled study of two fluid regimens in the initial management of septic shock in the emergency department. Pediatr Emerg Care 2008;24(10):647–655. DOI: 10.1097/PEC.0b013e31818844cf.
12. Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving sepsis campaign. International guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 2008;34(1):17–60. DOI: 10.1007/s00134-007-0934-2.
13. Taneja K, Kumar V, Anand R, Pemde HK. Normative data for IVC diameter and its correlation with the somatic parameters in Healthy Indian Children. Indian J Pediatr 2018;85(2):108–112. DOI: 10.1007/s12098-017-2440-z.
14. Silverman HJ, Peneranda R, Orens JB, Lee NH. Impaired beta-adrenergic receptor stimulation of cyclic adenosine monophosphate in human septic shock: association with myocardial hyporesponsiveness to catecholamines. Crit Care Med 1993;21(1):31–39. DOI: 10.1097/00003246-199301000-00010.
15. Scarpace PJ, Abrass IB. Desensitization of adenylate cyclase and down regulation of beta adrenergic receptors after in vivo administration of beta agonist. J Pharmacol Exp Ther 1982;223(2):327–331. PMID: 6127402.
________________________
© The Author(s). 2021 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.