CASE REPORT |
https://doi.org/10.5005/jp-journals-10071-23933 |
Massive Cerebral Air Embolism Causing Stroke Secondary to Pulmonary Tuberculosis
1,3Department of Anesthesiology and Intensive Care, Darsalam Clinic of Casablanca, Casablanca, Morocco
2Department of Anesthesiology and Intensive Care, CHU Ibn Rochd, Casablanca, Morocco
4Radiology Unit, Darsalam Clinic of Casablanca, Casablanca, Morocco
5Department of Radiology, CHU Ibn Rochd, Casablanca, Morocco
Corresponding Author: Abderrahmane Bouaggad, Department of Anesthesiology and Intensive Care, Darsalam Clinic of Casablanca, Casablanca, Morocco, Phone: +212 5 22 85 14 14, e-mail: darsalam.bouaggad@gmail.com
How to cite this article: Bouaggad A, Moussaoui M, Abassi O, Hassen S, Essodegui F. Massive Cerebral Air Embolism Causing Stroke Secondary to Pulmonary Tuberculosis. Indian J Crit Care Med 2021;25(8):942–944.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Cerebral air embolism due to pulmonary tuberculosis is an extremely rare cause of stroke. We report an unusual case of a presentation of cerebral air embolism likely due to pulmonary tuberculosis lesions during a severe cough. We discuss the relationship between the pulmonary tuberculosis and the occurrence of the cerebral air embolism. A 55-year-old man with lung tuberculosis suddenly experienced a nontraumatic loss of consciousness after a severe cough. The magnetic resonance imaging confirmed an ischemic stroke due to cerebral air embolism. The thoracic scan revealed tuberculosis with a parenchymatous cavity. Patients with intrapulmonary tuberculosis cavities should be strongly considered for surgical repair and should be warned about the risk of rupture of the cavity in the situation of increasing thoracic pressure.
Keywords: Cerebral air embolism, Pulmonary tuberculosis, Stroke.
INTRODUCTION
Cerebral air embolism is an extremely rare cause of stroke and the prognosis is seriously poor even on an appropriate, early diagnosis, and treatment. Cerebral air embolism secondary to pulmonary tuberculosis is rare with only a few cases being previously reported. Asymptomatic pulmonary tuberculosis lesions have not been reported in the literature as a cause of cerebral air embolism.1–4 Cerebral air embolism in patients with lung tuberculosis cavity is thought to be related to pressure change during sudden acute cough. In this study, we discuss the relationship between the pulmonary tuberculosis cavity and the occurrence of the cerebral air embolism during acute cough attack. The potential preventive role of lung tuberculosis cavity treatment is discussed.
This case refers to a patient who presented a massive ischemic stroke by cerebral air embolism due to asymptomatic pulmonary tuberculosis cavities.
CASE DESCRIPTION
A 55-year-old man with a weight of 76 kg and height of 178 cm, suddenly experienced a nontraumatic loss of consciousness after a cough attack at an upright position as reported by a witness. Two months prior, he was diagnosed with pulmonary tuberculosis and had been receiving antituberculosis treatment. On arrival, the patient was unconscious with a Glasgow coma scale score of 6, blood pressure of 169/89 mm Hg, regular heart rate of 85 beats/minute, respiratory rate of 18/minute, body temperature of 36.5°C, SaO2 of 82% at room air, and capillary blood sugar of 110 mg/dL. Auscultation of the lungs revealed an inspiratory crackle at the left lung field. After rapid intravenous access and oxygenation, the patient was sedated, the trachea was intubated, mechanical ventilation was initiated, and conservative treatment was maintained in the intensive care unit. Urgent computed tomography (CT) of the brain was performed and showed multiple air shadows in both hemispheres and the right cerebral vessels (Fig. 1). These lesions suggest air embolism. Twelve hours later, a magnetic resonance imaging (MRI) of the head was performed and showed a diffuse ischemic stroke in the right and left frontoparietal cortical areas and multiple hypointense signals, which were consistent with cerebral air embolism (Fig. 2).
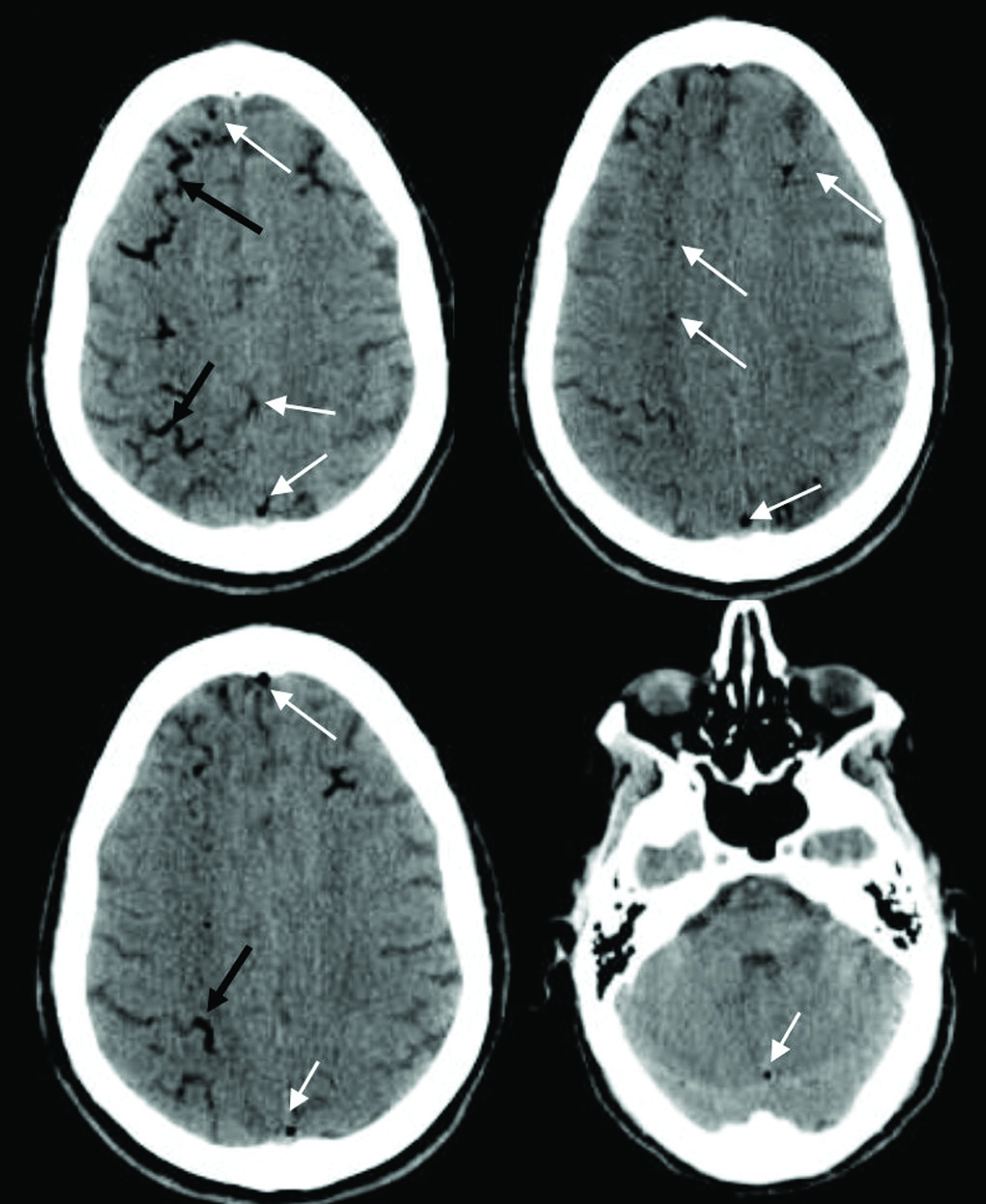
Fig. 1: Head CT revealed an air shadow in both hemispheres (White arrows), and in the right cerebral vessels (Black arrows). These lesions suggest air embolism
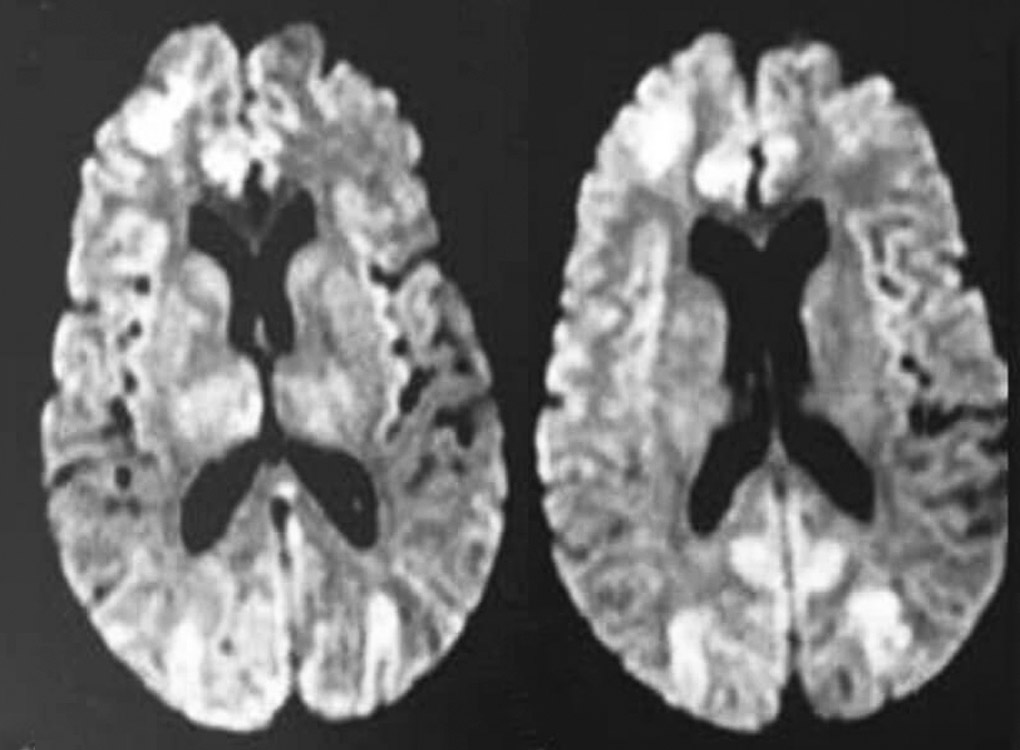
Fig. 2: MRI of the brain. DWI (diffusion-weighted image) demonstrates acute infarction in the right and left frontoparietal cortical areas and diffuse hypointense signals
The brain perfusion images were normal. The chest CT performed at the emergency department showed pulmonary tuberculosis cavity associated with the destruction of parenchyma in the left upper lobe (Fig. 3). There was no evidence of cerebral hemorrhage. The diagnosis of cerebral gas embolism after sudden cough was retained, and immediate treatment was started including 100% oxygen and cardiovascular support while the patient was placed in a supine position. The transthoracic echocardiography was performed, and no patent foramen ovale was found. A lumbar puncture was performed to seek for tuberculous meningoencephalitis. The cerebrospinal fluid analysis was normal. Hyperbaric oxygen therapy was considered, but the patient’s condition was too unstable to support transfer to the hyperbaric oxygen center. One day later, his neurological status deteriorated. The patient died on the fourth day with multiple organ failure. No autopsy was performed based on his family’s wishes.

Fig. 3: Chest computed tomography shows pulmonary cavity associated by destruction of parenchyma in the left upper lobe
DISCUSSION
The patient was diagnosed with cerebral air embolism due to pulmonary tuberculosis because no others causes of air embolism were identified. The patient in the present study developed cerebral air embolism during a sudden cough attack as reported by his family. The analysis of cerebrospinal fluid was performed and excluded tuberculous meningoencephalitis. Cerebral air embolism secondary to pulmonary barotrauma after a sudden cough is rare. In the literature, only three cases have described cerebral air embolism secondary to pulmonary tuberculosis latent lesions following cough,2 during an airline flight3 or following defecation.4 The physiopathology of gas entry to the systemic circulation remains unclear.1–4 The mechanism of cerebral air embolism associated with pulmonary cavities is explained by the elevation of intrathoracic pressure during sudden intense cough, leading the air into the systemic circulation and causing the systemic air embolism. The wall of tuberculosis cavities is generally a thin and fragile necrotic tissue.5,6 During acute cough attack, the pressure into cavity increased and may cause tears of its wall and air leaking into the systemic circulation. The arterial air embolism could be explained by (1) air passage via bronchial veins to systemic arterial circulation through patent foramen ovale, (2) gas flowed via pulmonary veins surrounding the lung cavities to left ventricle, and (3) gas migration into pulmonary arterial circulation through the pulmonary capillaries to systemic circulation. In our case, no persistent ovale foramen was found by echocardiography examination, and we suppose that pulmonary cavity was ruptured into pulmonary circulation. The mechanism of gas embolism into cerebral vessels may be explained by the upright position of the patient during the cough attack as reported by the witness. The prognosis of cerebral air embolism is extremely poor even when the volume of gas is small and injection of 2 mL of air in the cerebral arteries can be fatal.7 The immediate treatment of suspected cerebral air embolism consists of administering 100% oxygen and placing the patient in the supine position. Hyperbaric oxygen treatment within 4 to 6 hours is considered useful as it could decrease bubble size and increase cerebral tissue oxygenation. In the present case, hyperbaric oxygen therapy is not provided due to the rapid deterioration of cardiovascular condition. Patients with known pulmonary tuberculosis sequelae should be advised of the risk of the systemic air embolism in a situation increasing lung cavity rupture.8 Surgical treatment as a pulmonary resection could be discussed in such cases with tuberculosis lung cavern. Pulmonary lobectomy in the case of rupture giant pulmonary bulla complicated by cerebral air embolism has been reported.9 Tuberculosis is more common in developing countries and can lead to life-threatening complications: lung cavity, chronic respiratory failure, massive hemoptysis, and cerebral air embolism. Surgical therapy options should be considered in the panel of the treatment in such cases.
CONCLUSION
Some lessons through this case could be helpful to avoid such complications: (1) In situation of cerebral air embolism, a pulmonary lesion should be sought out and chest CT performed and (2) we should consider the surgical repair option in case of tuberculosis parenchymatous cavity sequelae to avoid this kind of fatal complication.
ORCID
Abderrahmane Bouaggad https://orcid.org/0000-0002-1530-4117
REFERENCES
1. Ferry T, Argaud L, Delafosse B, Robert D. Inactive tuberculosis cavity responsible for fatal cerebral air embolism. Intensive Care Med 2006;32(4):622–623. DOI: 10.1007/s00134-006-0091-z.
2. Rodriguez JF, Liron L, Chambost M, Combe C. Cerebral gas embolism after cough fit. Ann Fr Anesth Reanim 2005;24(1):64–67. DOI: 10.1016/j.annfar.2004.10.023.
3. Jung HS, Jeong HW, In HS. Cerebral air embolism in a patient with a tuberculous-destroyed lung during commercial air travel: a case report. J Korean Soc Radiol 2011;65(2):109–112. DOI: 10.3348/jksr.2011.65.2.109.
4. Oh JY, Park DW, Hahm CK, Park CK, Lee SR, Lee Y. A cerebral air embolism that developed following defecation in a patient with extensive pulmonary tuberculosis: a case report. J Korean Soc Radiol 2010;63(4):307–310. DOI: 10.3348/jksr.2010.63.4.307.
5. Togo M, Hoshi T, Matsuoka R, Imai Y, Kohara N. Multiple small hemorrhagic infarcts in cerebral air embolism: a case report. BMC Res Notes 2017;16(10):599. DOI: 10.1186/s13104-017-2925-x.
6. Farshchi Zarabi S, Parotto M, Katznelson R, Downar J. Massive ischemic stroke due to pulmonary barotrauma and cerebral artery air embolism during commercial air travel. Am J Case Rep 2017;18:660–664. DOI: 10.12659/AJCR.903354.
7. Ho A M, Ling E. Systemic air embolism after lung trauma. Anesthesiology 1999;90(2):564–475. DOI: 10.1097/00000542-199902000-00033.
8. Almeida FA, DeSouza BX, Meyer T, Gregory S, Greenspon L. Intrapulmonary bronchogenic cyst and cerebral gas embolism in an aircraft flight passenger. Chest 2006;130(2):575–577. DOI: 10.1378/chest.130.2.575.
9. Gudmundsdottir JF, Geirsson A, Petur Hannesson P, Gudbjartsson T. Massive ischemic stroke due to pulmonary barotrauma and cerebral artery air embolism during commercial air travel. BMJ Case Rep 2015;2015. DOI: 10.1136/bcr-2014-208159. pii: bcr2014208159.
________________________
© The Author(s). 2021 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.