ORIGINAL ARTICLE |
https://doi.org/10.5005/jp-journals-10071-23962 |
Ultrasonographic Assessment of Diaphragmatic Inspiratory Amplitude and Its Association with Postoperative Pulmonary Complications in Upper Abdominal Surgery: A Prospective, Longitudinal, Observational Study
1,4–6Department of Anaesthesiology, Cancer Institute (WIA), Chennai, Tamil Nadu, India
2Department of Anaesthesiology, Pain and Palliative Care, Cancer Institute (WIA), Chennai, Tamil Nadu, India
3Department of Radiology, Cancer Institute (WIA), Chennai, Tamil Nadu, India
Corresponding Author: Prasanna V Vanamail, Department of Anaesthesiology, Cancer Institute (WIA), Chennai, Tamil Nadu, India, Phone: +91 9487945184, e-mail: prasna.vani@gmail.com
How to cite this article: Vanamail PV, Balakrishnan K, Prahlad S, Chockalingam P, Dash R, Soundararajan DK. Ultrasonographic Assessment of Diaphragmatic Inspiratory Amplitude and Its Association with Postoperative Pulmonary Complications in Upper Abdominal Surgery: A Prospective, Longitudinal, Observational Study. Indian J Crit Care Med 2021;25(9):1031–1039.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Background: Diaphragmatic dysfunction following upper abdominal surgery is less recognized due to a lack of diagnostic modality for bedside evaluation. We used point-of-care ultrasound to evaluate the diaphragmatic inspiratory amplitude (DIA) in upper abdominal surgery for cancer. Our primary hypothesis was DIA would be reduced in the immediate postoperative period in patients with postoperative pulmonary complications (PPCs). Our aim was to identify an optimal cutoff of DIA for the diagnosis of PPCs.
Methods: We conducted a prospective, observational study in patients aged 18–75 years undergoing elective, upper abdominal oncological surgeries under combined general and epidural anesthesia. Ultrasound evaluation of the diaphragm was done by measuring the DIA in the right and left hemidiaphragms during quiet and deep breathing on the day before surgery and postoperative days (PODs) 1, 2, and 3. Patients were followed up for PPCs until POD 7. The linear mixed-effects model examined the association between DIA and PPCs and other perioperative factors. Receiver-operating characteristics analysis was done to determine the optimal cutoff of DIA in diagnosing PPCs.
Results: DIA measured in the 162 patients showed a significant decrease in their absolute values postoperatively from its preoperative baseline measurement. This decrease in DIA was significantly associated with PPC [right hemidiaphragm, β = −0.17, 95% confidence interval (CI) −0.31 to −0.02, p = 0.001 during quiet breathing; left hemidiaphragm, β = −0.24, 95% CI = −0.44 to −0.04, p = 0.018 and β = −0.40, 95% CI = −0.71 to −0.09, p = 0.012 during quiet and deep breathing, respectively]. A cutoff value of DIA of left hemidiaphragm at 1.3 cm during quiet breathing and 1.6 cm during deep breathing had a sensitivity of 77 and 75%, respectively, in their ability to diagnose PPCs [left hemidiaphragm quiet breathing, area under the curve (AUC): 0.653, 95% CI 0.539–0.768, p = 0.015; left hemidiaphragm deep breathing, AUC: 0.675, 95% CI 0.577–0.773, p = 0.007].
Conclusion: Following upper abdominal surgery, the DIA is decreased and associated with PPCs. DIA of left hemidiaphragm less than 1.3 cm during quiet breathing and 1.6 cm during deep breathing has a sensitivity of 77 and 75%, respectively, in diagnosing PPCs following upper abdominal surgery.
Keywords: Diaphragm excursion, Diaphragmatic dysfunction, Diaphragmatic inspiratory amplitude, Gastrectomy, Pancreaticoduodenectomy, Pneumonia, Postoperative, Pulmonary complications, Ultrasound, Upper abdominal surgery.
HIGHLIGHTS
Perioperative diaphragmatic inspiratory amplitude (DIA) was assessed using point-of-care ultrasound in patients who underwent upper abdominal surgery for cancer. The diaphragmatic movement was reduced after surgery, and it was significantly associated with postoperative pulmonary complications (PPCs). DIA of the left hemidiaphragm less than 1.3 cm during quiet breathing and 1.6 cm during deep breathing has good sensitivity in diagnosing PPCs following upper abdominal surgery. Ultrasound assessment of the diaphragm is a valuable tool in monitoring postoperative diaphragmatic dysfunction.
INTRODUCTION
The diaphragm is the principal muscle of respiration. Diaphragmatic dysfunction (DD) is an underestimated cause of respiratory impairment in postsurgical patients.1 Upper abdominal surgeries increase the risk of postoperative DD.2 This is purported due to reflex inhibition of phrenic motor output from visceral afferents.3 Historically, monitoring for DD has been onerous due to the need for complex equipment and expertise as fluoroscopy, transdiaphragmatic pressure measurement, and computerized tomography. Point-of-care ultrasonogram (USG) is a promising modality for real-time monitoring of DD.
Monitoring perioperative respiratory muscle dysfunction is at an incipient stage. Diaphragmatic movement correlates well with postoperative vital capacity and lung compliance.4,5 Impairment of these lung function parameters is fundamental in the pathogenesis of postoperative pulmonary complication (PPC). Patients with PPCs have increased morbidity, mortality, healthcare resource utilization, and hospitalization expenses, and in cancer patients, it can delay return to intended oncological therapy.6–8
The perioperative changes in diaphragmatic function and their association with PPCs have not been investigated in upper abdominal surgeries. This study aims to measure the diaphragmatic inspiratory amplitude (DIA) by ultrasound preoperatively and on postoperative days (PODs) 1–3 in patients undergoing upper abdominal surgery for cancer. Our primary objective is to quantify the decrease in the absolute values of DIA postoperatively from its preoperative value and identify an optimal cutoff for identifying patients with PPCs from others. Our secondary objective is to identify the various perioperative factors affecting DIA.
METHODS
The institutional ethics committee approved this study (Reference Number IEC/2017/11). We obtained written informed consent from all participants. This manuscript adheres to the Strengthening the Reporting of Observational Studies in Epidemiology statement guidelines.9
Study Design
We conducted a prospective, observational study at a tertiary cancer hospital over 2 years—September 2017 to March 2019.
Inclusion Criteria
We included patients between 18 and 75 years of age who belonged to the American Society of Anesthesiologists Physical Status (ASA-PS) Classifications 1 and 2 and underwent elective, open, upper abdominal cancer surgeries under general anesthesia (GA) with epidural analgesia.
Exclusion Criteria
We excluded vulnerable subjects, ASA-PS three or four patients, and patients who underwent emergency surgeries, palliative procedures, laparoscopic surgeries, patients on steroids preoperatively, patients with pulmonary diseases, those planned for elective postoperative ventilation, patients who were re-intubated during the first 7 days postoperatively for a nonpulmonary cause, and patients who experienced ineffective epidural analgesia.
Patient Recruitment
Patients were recruited at the time of their preoperative assessment at least 2 weeks before surgery. Smokers were advised to stop smoking. All patients were started on incentive spirometry. Preoperative USG was done to measure the DIA on the right hemidiaphragm during quiet (RQ) and deep breathing (RD) and on the left hemidiaphragm during quiet (LQ) and deep breathing (LD) in the supine position.
Measurement Technique
All examinations were performed using a MyLab™ 25 Gold, 3.5 MegaHertz phased array probe (Esaote, Genova, Italy). A senior radiologist with 25 years of experience trained the investigator who performed the USG and periodically supervised the USG examinations. This same investigator performed the assessment preoperatively and postoperatively. The investigator stood on the right side of the patient.
Right Hemidiaphragm Assessment
The ultrasound probe was placed on the right anterior chest wall between the midclavicular line and anterior axillary line at the ninth intercostal space. The probe was placed in craniocaudal orientation and directed cephalad, medially, and dorsally. The brightness mode was used to acquire the diaphragm image. The probe was adjusted to obtain a continuous trace of the diaphragm against the acoustic window of the liver with the confluence of the portal vein in view. By directing the ultrasound beam in this direction, we were able to visualize the posterior third of the diaphragm in the anterior subcostal view, where diaphragmatic movement is greatest.10
Motion mode was then selected, and the cursor was placed perpendicular to the diaphragm’s movement. A sweep speed of 10/second was set during examination in this mode. The sine wave obtained from the diaphragm’s movement to and fro about the probe during a respiratory cycle was frozen. The distance from the sine wave’s trough to the highest echogenic line and the sine wave’s peak to the highest echogenic line was measured (Fig. 1). The DIA was calculated from the difference between these two values. Brightness mode imaging was then retaken, and the patient was requested to take a deep breath and then exhale. If the rib shadows interfered with the diaphragm’s imaging, the probe was moved caudally until a good diaphragmatic excursion was obtained without interference. The average of three measurements was taken for absolute values of DIA during quiet and deep breathing separately.

Figs 1A to D: M-mode projection of the ultrasound beam and measurement of diaphragmatic inspiratory amplitude in (A) RQ; (B) RD; (C) LQ; and (D) LD. RQ, right hemidiaphragm during quiet breathing; RD, right hemidiaphragm during deep breathing; LQ, left hemidiaphragm during quiet breathing; and LD, left hemidiaphragm during deep breathing. Time on abscissa with a sweep speed of 10 seconds and distance on the ordinate. Ventral skin echoes (1); M-mode beam (green line); diaphragm echoes (arrow); liver parenchyma (L); portal vein (PV); and spleen parenchyma (S)
Left Hemidiaphragm Assessment
The probe was placed on the lateral chest wall on the left side between the anterior and posterior axillary lines. The probe was placed in craniocaudal orientation and directed cephalad, medially, and dorsally. Motion mode was then applied, and the absolute values of DIA were measured as detailed above. If the excursions could not be obtained without interference from the rib shadows, while the images were saved, these measurements were considered missing values.
Management of Anesthesia
A thoracic epidural block was performed preoperatively at a level of thoracic vertebrae between 6 and 10. A dermatomal analgesia level was assessed by checking for cold perception following a test dose of local anesthesia before induction of GA. Patients were induced with 2 µg/kg fentanyl and 2–2.5 mg/kg propofol, with the neuromuscular block being achieved with 0.1 mg/kg vecuronium. All patients received controlled ventilation with a 6 mL/kg tidal volume and a positive end-expiratory pressure of 5 cm water. GA was maintained with sevoflurane, nitrous oxide, and 50% oxygen, targeted to minimum alveolar concentration 0.8–1. The neuromuscular block was maintained with intermittent doses of 0.25 mg/kg vecuronium guided by neuromuscular monitoring. An epidural infusion of 0.125% bupivacaine at 3–6 mL/hour was started before the incision and continued throughout the surgery. All patients received an epidural bolus dose of 50 µg/kg morphine every 8 hours intraoperatively. At the end of the surgery, the neuromuscular block was reversed with 30–50 µg/kg neostigmine and 10 µg/kg glycopyrrolate. An epidural infusion of 0.125% bupivacaine 3–6 mL/hour was continued until the second POD, and 50 µg/kg morphine was continued epidurally every 8 hours until the third POD. An upper midline incision was used for all surgeries, except for hepatobiliary surgeries (HBS), which used a right-sided subcostal incision.
Postoperative DIA Assessment
USG examinations were performed in the supine position. DIA was measured with an epidural infusion of bupivacaine on flow and only if the patient’s pain score was <3, as assessed by a visual analog scale. If the score was >3, patients were given 15 mg/kg paracetamol intravenously and reassessed 30 minutes later. All patients were mobilized on the second POD.
Missing Values
USG DIA values that were not obtained any day due to difficulties with visualization were considered missing values. If the DIA was not obtained for longer than a day for any patient, either due to technical difficulties or the requirement of continuous ventilator support, they were excluded from further analysis. In patients requiring intermittent noninvasive ventilation, the USG assessment of DIA was performed when the patients were off noninvasive ventilation and comfortable. For patients who received continuous noninvasive ventilation support and those who received invasive ventilation, a USG assessment was not done on that day.
Postoperative Pulmonary Complications
Diagnosis of PPC was based on the European Perioperative Clinical Outcome guidelines, and their severity was graded based on the Clavien–Dindo classification.11,12 Patients were followed up for the development of PPCs for 7 days. PPCs were assessed by an independent investigator who did not know the USG DIA measurements.
We grouped transhiatal esophagectomy and extended total gastrectomy as proximal surgeries as they involve considerable handling of the diaphragm. Pancreaticoduodenectomy, hepatectomy, liver metastasectomy, and radical cholecystectomy were grouped together as hepatobiliary surgeries (HBS).
Sample Size Calculation
In a study on cardiac surgeries, preoperative diaphragmatic thickening fraction was able to predict PPC. We estimated our sample size based on the preoperative DIA, expecting preoperative DIA to be impaired in those patients who subsequently developed pulmonary complications.13 In our pilot study, the mean ± standard deviation (SD) of preoperative RD DIA measurements for the No-PPC and PPC-group were 4.16 ± 1.35 cm and 3.6 ± 1.02 cm, respectively. The correlation coefficient between baseline and follow-up was 0.5. We presumed similar observations would be made for repeated measures using a mixed model study (one measurement of baseline and three follow-up measures), and given the case to control ratio of 1:5, an adequate sample size for 80% power with a 5% level of significance was calculated to be 25 cases and 125 controls.
Statistical Analysis
We performed the data analysis using Stata version 16.0. To test the normality assumptions of continuous variables, we used the Kolmogorov–Smirnov test. Descriptive measures, such as mean (standard deviation) and range values, were presented for normally distributed data. Considering the outcome measures of RQ, RD, LQ, and LD at different time points after surgery, we used the linear mixed random effect models to analyze the intervention’s effect. The same individual measures were modeled as random effects in a random intercept model, while surgery was modeled as a fixed effect. The model’s outcome variables were RQ, RD, LQ, and LD measurements. The measurement times were divided into day 0, day 1, day 2, and day 3. The main factors were the day of measurement, gender, and type of surgery. Other study variables were taken as covariates in the analysis. While observing overall significant variation between the primary factors and interaction factors, we used the Bonferroni method for pairwise comparison of estimated marginal means to correct for the potential increase of the probability of type I error. A two-sided probability of p <0.05 for Wald chi-square was considered statistical significance for all the model parameters estimation. Receiver-operating characteristics (ROC) analysis was performed, and the area under the curve (AUC) was calculated on the various DIA measurements to evaluate its ability to diagnose PPCs. The cutoff with the maximum value of Youden’s index, which is defined as (sensitivity + specificity −1), was considered as the optimal cutoff.
RESULTS
DIA was assessed in 191 patients preoperatively, and 162 patients were included in the final analysis (Flowchart 1). No patients were excluded due to ineffective epidural analgesia or high pain score. The baseline demographic data, details of the surgery, and PPCs are shown in Table 1. PPCs encountered in our study were pneumonia, respiratory failure, and acute respiratory distress syndrome. The distribution of types of surgeries is depicted in Figure 2.
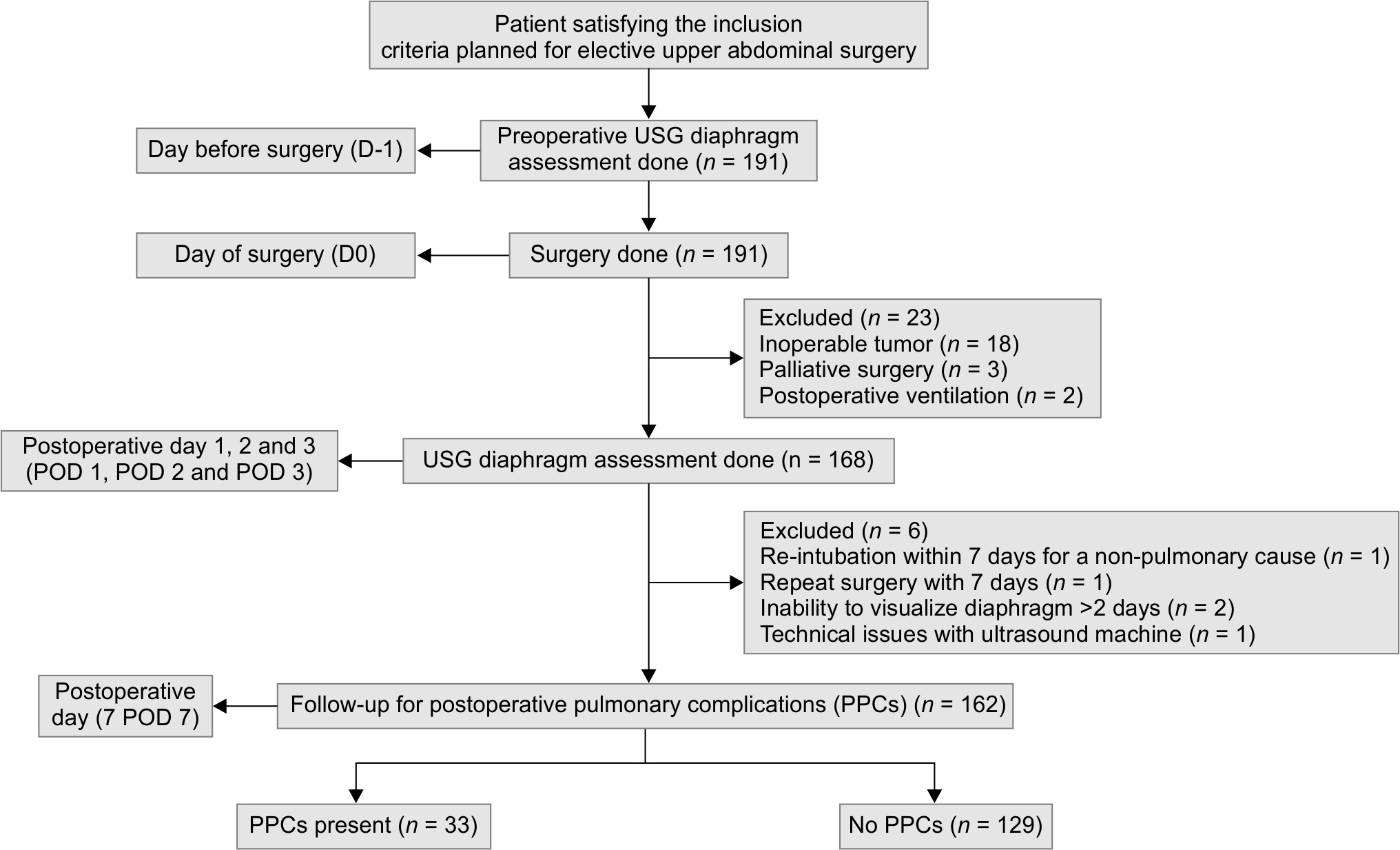
Flowchart 1: Outline of patient recruitment and follow-up for outcome assessment

Fig. 2: Distribution of surgeries
| Parameter | Value |
|---|---|
| Demographic characteristics | |
| Age, years | 55.9 ± 11 |
| Male gender | 101 (62.3) |
| Height, cm | 158.7 ± 8.9 |
| Body mass index, kg/m2 | 22.2 ± 4.6 |
| Risk factors | |
| Charlson’s comorbidity index | 2 (2–3) |
| Smoking history present | 37 (22.8) |
| Pack years of smokers | 5 ± 15 |
| Time since the cessation of smoking, months | 7.2 ± 29 |
| NRS 2002 score for malnutrition* | 4 (3–5) |
| Laboratory parameters | |
| Hemoglobin, gm/dL | 11 ± 2 |
| Total WBC count, L−1 | 7.6 ± 3.4* 109 |
| Preoperative serum albumin, gm/dL | 3.3 ± 0.6 |
| Intraoperative variables | |
| Duration of surgery, minutes | 335.8 ± 130.5 |
| Intraoperative tidal volume, mL/kg | 8.4 ± 2 |
| Intraoperative PEEP, cm water | 5 ± 0.7 |
| Total dose of vecuronium, mg/kg hr | 0.04 ± 0.02 |
| Lowest intraoperative temperature, Celsius | 35.1 ± 0.8 |
| Level of epidural catheter placement* | 9 (8–9) |
| Intraoperative blood loss, mL | 570 ± 789 |
| Details of PPC | |
| Patients with PPCs | 33 (20.4) |
| Patients who required NIV | 18 (11.1) |
| Patients who required intubation | 7 (4.3) |
| Mortality due to PPCs | 4 (2.5) |
DIA on the Right Hemidiaphragm during Quiet Breathing (DIA RQ)
We observed that the absolute value of DIA in RQ decreased over the three PODs across the entire cohort compared to its preoperative value (p <0.001). After adjusting for the various perioperative variables, there was a significant association between decreased DIA in RQ and PPC [β = −0.17, 95% confidence interval (CI) −0.31 to −0.02, p = 0.03] (Table 2). However, there was no significant difference in the estimated marginal mean of the DIA in RQ between patients with and without PPC at any day of the measurement (Fig. 3A). Males had significantly higher DIA than females (β = 0.2, 95% CI 0.03–0.36, p = 0.023). The DIA in RQ in patients who underwent HBS was significantly less compared to the other surgeries (β = −0.2, 95% CI −0.37 to −0.04, p = 0.017) (Table 2). Comparison of day-wise mean DIA between HBS and other surgeries did not show a significant difference (Fig. 4A). None of the other covariates affected the DIA RQ outcome (Electronic Supplementary Table S1).

Figs 3A to D: Estimated marginal means and 95% confidence intervals of DIA of (A) RQ; (B) LQ; (C) RD, and (D) LD in patients who developed postoperative pulmonary complications and in others. DIA, diaphragmatic inspiratory amplitude; RQ, right hemidiaphragm during quiet breathing; RD, right hemidiaphragm during deep breathing; LQ, left hemidiaphragm during quiet breathing; LD, left hemidiaphragm during deep breathing; PPC, postoperative pulmonary complication
| DIA RQ | DIA RD | |||||
|---|---|---|---|---|---|---|
| Variables | B | 95% CI | p value | β | 95% CI | p value |
| Day | ||||||
| 0 | Ref. | Ref. | ||||
| 1 | −0.43 | −0.52 to −0.34 | 0.000 | −2.00 | −2.21 to −1.79 | 0.000 |
| 2 | −0.35 | −0.45 to −0.26 | 0.000 | −1.96 | −2.17 to −1.75 | 0.000 |
| 3 | −0.25 | −0.34 to −0.15 | 0.000 | −1.83 | −2.04 to −1.61 | 0.000 |
| No PPC | Ref. | Ref. | ||||
| PPC present | −0.17 | −0.31 to −0.02 | 0.030 | −0.27 | −0.58 to 0.03 | 0.076 |
| Gender Female | Ref. | Ref. | ||||
| Male | 0.20 | 0.03−0.36 | 0.023 | 0.24 | −0.11 to 0.59 | 0.184 |
| Other surgeries | Ref. | Ref. | ||||
| Proximal surgeries | 0.08 | −0.06 to 0.23 | 0.254 | 0.10 | −0.21 to 0.40 | 0.534 |
| Other surgeries | Ref. | Ref. | ||||
| Hepatobiliary surgeries | −0.20 | −0.37 to −0.04 | 0.017 | −0.16 | −0.59 to 0.26 | 0.457 |
| Age | 0.01 | 0.00 to 0.02 | 0.048 | |||
| D1_intake | 0.37 | 0.00 to 0.74 | 0.048 | |||

Figs 4A to D: Estimated marginal means and 95% confidence intervals of DIA of (A) RQ; (B) LQ; (C) RD, and (D) LD in patients who underwent hepatobiliary surgeries and in those who had other upper abdominal surgeries. *denotes p value <0.05. DIA, diaphragmatic inspiratory amplitude; RQ, right hemidiaphragm during quiet breathing; RD, right hemidiaphragm during deep breathing; LQ, left hemidiaphragm during quiet breathing; LD, left hemidiaphragm during deep breathing; HBS, hepatobiliary surgeries
DIA on the Left Hemidiaphragm during Quiet Breathing (DIA LQ)
The absolute value of DIA in LQ was found to be significantly less on all PODs as compared to its preoperative value (p <0.001). The association of covariates with DIA LQ is presented in Table 3 and Electronic Supplementary Table S3. The DIA among patients with PPC was significantly less than other patients (β = 0.24, 95% CI = −0.44 to −0.04, p = 0.018). Despite this, no significant difference was observed in mean DIA between patients with and without PPC at individual days of measurement (Fig. 3B). The DIA LQ was observed to be significantly increased among the patients who had HBS compared to those who had other surgeries (p = 0.03) (Table 3). All patients had a decrease in postoperative DIA in LQ, which was not significant between the HBS group and other surgery groups at each day of measurement (Fig. 4B).
| DIA LQ | DIA LD | |||||
|---|---|---|---|---|---|---|
| Variables | β | 95% CI | p value | β | 95% CI | p value |
| Day | ||||||
| 0 | Ref. | Ref. | ||||
| 1 | −0.46 | −0.57 to −0.35 | 0.000 | −1.58 | −1.76 to −1.41 | 0.000 |
| 2 | −0.41 | −0.52 to −0.29 | 0.000 | −1.64 | −1.82 to −1.46 | 0.000 |
| 3 | −0.44 | −0.56 to −0.32 | 0.000 | −1.40 | −1.58 to −1.22 | 0.000 |
| No PPC | Ref. | Ref. | ||||
| PPC present | −0.24 | −0.44 to −0.04 | 0.018 | −0.40 | −0.71 to −0.09 | 0.012 |
| Gender Female | Ref. | Ref. | ||||
| Male | 0.16 | −0.03 to 0.35 | 0.093 | 0.35 | 0.05 to 0.66 | 0.021 |
| Other surgeries | Ref. | Ref. | ||||
| Proximal surgeries | −0.08 | −0.24 to 0.09 | 0.350 | −0.19 | −0.46 to 0.08 | 0.161 |
| Other surgeries | Ref. | Ref. | ||||
| Hepatobiliary surgeries | 0.20 | 0.02 to 0.39 | 0.030 | 0.15 | −0.15 to 0.44 | 0.321 |
DIA on the Right Hemidiaphragm during Deep Breathing (DIA RD)
The absolute value of DIA in RD was also significantly (p <0.001) reduced from the preoperative day to PODs in all patients (p <0.001) (Table 2). PPC was not significantly associated with DIA in RD (β = −0.27, 95% CI = −0.58–0.03, p = 0.076). Among the other covariates, age and fluid intake on POD 1 had a significant effect on DIA (Table 2). The effect of other covariates was not significant (Electronic Supplementary Table S2). Day-specific marginal mean did not show a significant decrease in patients with PPCs (Fig. 3C). The mean DIA in RD of patients who underwent HBS was significantly less than those who had other upper abdominal surgeries on POD 1 and POD 2 (p <0.05) (Fig. 4C).
DIA on the Left Hemidiaphragm during Deep Breathing (DIA LD)
The absolute value of DIA in LD also showed a significant decline (p <0.001) postoperatively from its preoperative observation. The mean DIA among males was significantly higher compared to females (β = 0.35, 95% CI = 0.05–0.66, p = 0.021). DIA was significantly decreased in patients with PPCs (β = −0.4, 95% CI = −0.71 to −0.09, p = 0.012) (Table 3). However, the day-specific measurements did not show a significant difference between patients with PPCs and others (Fig. 3D). There was no significant association between HBS and DIA (p = 0.321). The difference in DIA between patients who had HBS and those who had other surgeries on each day of measurement was not significant (Fig. 4D). None of the other covariates affected the DIA LD outcome (Electronic Supplementary Table S4).
ROC Analysis
The ROC curve was plotted to identify a cutoff value of DIA for the prediction of PPCs. (Fig. 5) According to the ROC curve, DIA LQ on POD 3 had an AUC of 0.653, 95% CI 0.538–0.768, and p value 0.015. At a cutoff of 1.3 cm, the sensitivity was 77 % and specificity was 50% for the diagnosis of PPCs. DIA LD on POD 3 had an AUC of 0.675, 95% CI 0.577–0.773, and p value 0.007. At a cutoff of 1.6 cm, DIA LD on POD 3 was able to diagnose PPCs with 75% sensitivity and 63% specificity. The remaining DIA measurements had an AUC of 0.4–0.59, which were also not statistically significant.

Figs 5A and B: Receiver operating characteristics of (A) Left hemidiaphragm during quiet breathing on D3 (AUC of 0.653, 95% CI 0.538–0.768, and p value 0.015) and (B) Left hemidiaphragm during deep breathing (AUC of 0.675, 95% CI 0.577–0.773, and p value 0.007). AUC, area under the curve
DISCUSSION
Our study demonstrates that in patients undergoing upper abdominal surgery for cancer, reduced diaphragmatic excursion postoperatively is associated with PPCs. Patients who had HBS have greater right hemidiaphragmatic impairment with preserved left hemidiaphragmatic excursion in the postoperative period. At the identified cutoffs of left hemidiaphragmatic excursions, we were able to predict PPC with good sensitivity and specificity.
Associations of impaired diaphragmatic function and PPCs are seen following cardiac, thoracic, and pelvic laparoscopic surgeries.13–15 Our findings are relevant in that we demonstrate a significant association between DIA and PPCs following upper abdominal surgery.
In agreement with our findings, an observational study on patients who underwent open liver lobectomy found that the DIA was reduced by 60% from the preoperative values on POD 1 and 2. The DIA improved by 30% on POD 7 from their POD 1 and 2 values. DIA was also observed to have a significant positive correlation with concomitantly measured vital capacity with spirometry.4 A decrease in postoperative DIA with a significant correlation with inspiratory capacity was also seen following open and laparoscopic cholecystectomy.16
The incidence of PPCs increases linearly with the proximity of the surgical incision to the diaphragm, suggesting DD being a predominant cause of PPCs.17 Furthermore, expiratory muscle tone is increased during expiration, pushing the diaphragm high into the rib cage, causing a restrictive lung function with reduced functional residual capacity, tidal volume, and vital capacity.18 This restrictive pattern gets exacerbated in a postoperative patient due to incisional pain and causes a redistribution of pulmonary perfusion and gas exchange abnormalities.19,20 Besides, diaphragmatic movement is responsible mainly for ventilation of lower lung fields where atelectasis and infection frequently occur.21
We predefined the types of surgeries into HBS and others to evaluate the effect of these surgeries on DIA. HBS also result in bilateral impairment of DIA despite the surgical insult being predominantly to the right hemidiaphragm. Afferents to the phrenic nerve are stimulated by the pressure on hepatic veins, parenchyma, and inferior vena cava and can also inhibit the intercostal muscles via a supraspinal mechanism.22 Left hemidiaphragmatic DIA was affected to a lesser extent than right hemidiaphragmatic DIA in these patients. Local irritation from extensive manipulation of abdominal viscera, fluid collections, and drain tubes can inhibit diaphragm action in addition to reflex inhibition.23
All our patients received thoracic epidural block with bupivacaine at concentrations of 0.1–0.125%. Motor output of phrenic nerve improved after thoracic epidural block with bupivacaine at a concentration of 0.5%.24 Such higher concentrations are not practical in postoperative patients due to the risk of intercostal muscle weakness and hypotension. The improvement in diaphragmatic activity was not seen with epidural opioids despite adequate analgesia.25 This indicates the presence of factors other than nociception as contributors to DD.
ROC analysis shows a good sensitivity in the prediction of PPCs from POD 3 DIA of the left hemidiaphragm during quiet and deep breathing. The onset of PPCs in our patients was seen at a median of 3 days after surgery. This explains the predictive ability of POD 3 DIA measurements. Right hemidiaphragmatic impairment from local irritation in HBS confounds the effect of PPC on DIA. This could explain its low AUC.
In a longitudinal study, such as ours, collapsing repeated measures to simple summaries of each parameter can mask potential interactions present. Analyses of longitudinal data require statistical methods that account for the correlations between repeated observations within an individual.26 Failure to consider these correlations cause invalid data interpretation. We make a comprehensive assessment of diaphragmatic excursion, adjusting for all the factors that can influence it in a perioperative milieu.
LIMITATIONS
Left-sided measurements had missing values in our study due to technical difficulties while acquiring the ultrasound image. This difficulty has been reported by others too.10 We did not perform concomitant spirometry. The supine position is associated with a reduction in forced vital capacity of 0.17 L compared to the Fowler position (p= 0.001).27 Despite it, the evaluation of diaphragm in supine position has less side-to-side variability, better reproducibility, and better correlation with inspiratory capacity.10 Finally, intra-abdominal pressure, an essential factor affecting the diaphragm, was not measured.28
CONCLUSION
DIA of left hemidiaphragm less than 1.3 cm during quiet breathing and 1.6 cm during deep breathing, measured on third postoperative day, has a sensitivity of 77 and 75% respectively, in diagnosing PPCs following upper abdominal surgery.
ORCID
Prasanna V Vanamail https://orcid.org/0000-0002-0217-1052
Kalpana Balakrishnan https://orcid.org/0000-0003-3121-0230
Sarojini Prahlad https://orcid.org/0000-0002-8740-7799
Punitha Chockalingam https://orcid.org/0000-0003-2833-1277
Radhika Dash https://orcid.org/0000-0001-9847-907X
Dinesh K Soundararajan https://orcid.org/0000-0003-1829-5508
SUPPLEMENTARY MATERIAL
All the supplementary material from Supplementary 1–4 tables are available online on the website of www.IJCCM.org
REFERENCES
1. Nason LK, Walker CM, Mcneeley MF, Burivong W, Fligner CL, David Godwin J. Imaging of the diaphragm: anatomy and function. Radiographics 2012;32(2):E51–E70. DOI: 10.1148/rg.322115127.
2. Lomauro A. Should the diaphragm be evaluated after abdominoplasty? J Bras Pneumol 2019;45(3):e20190146. DOI: 10.1590/1806-3713/e20190146.
3. Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med 2003;168(1):10–48. DOI: 10.1164/rccm.2206020.
4. Kim SH, Na S, Choi J-S, Na SH, Shin S, Koh SO. An evaluation of diaphragmatic movement by M-Mode sonography as a predictor of pulmonary dysfunction after upper abdominal surgery. Anesth Analg 2010;110(5):1349–1354. DOI: 10.1213/ANE.0b013e3181d5e4d8.
5. Kim K, Jang DM, Park JY, Yoo H, Kim HS, Choi WJ. Changes of diaphragmatic excursion and lung compliance during major laparoscopic pelvic surgery: a prospective observational study. PLoS One 2018;13(11):1–9. DOI: 10.1371/journal.pone.0207841.
6. Gropper MA. Postoperative respiratory muscle dysfunction: only the strong survive. Anesthesiology 2013;118(4):783–784. DOI: 10.1097/ALN.0b013e318288823b.
7. Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004;199(4):531–537. DOI: 10.1016/j.jamcollsurg.2004.05.276.
8. Suzuki S, Kanaji S, Matsuda Y, Yamamoto M, Hasegawa H, Yamashita K, et al. Long-term impact of postoperative pneumonia after curative gastrectomy for elderly gastric cancer patients. Ann Gastroenterol Surg 2018;2(1):72–78. DOI: 10.1002/ags3.12037.
9. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370(9596):1453–1457. DOI: 10.1016/S0140-6736(07)61602-X.
10. Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle and Nerve 2013;47(3):319–329. DOI: 10.1002/mus.23671.
11. Jammer I, Wickboldt N, Sander M, Smith A, Schultz MJ, Pelosi P, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Artic Eur J Anaesthesiol 2015;32(2):88–105. DOI: 10.1097/EJA.0000000000000118.
12. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Ann Surg 2004;240(2):205–213. DOI: 10.1097/01.sla.0000133083.54934.ae.
13. Lerolle N, Guérot E, Dimassi S, Zegdi R, Faisy C, Fagon JY, et al. Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest 2009;135(2):401–407. DOI: 10.1378/chest.08-1531.
14. The LAS VEGAS investigators. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS – an observational study in 29 countries. Eur J Anaesthesiol 2017;34(8):492–507. DOI: 10.1097/EJA.0000000000000646.
15. Spadaro S, Grasso S, Dres M, Fogagnolo A, Dalla Corte F, Tamburini N, et al. Point of care ultrasound to identify diaphragmatic dysfunction after thoracic surgery. Anesthesiology 2019;131(2):266–278. DOI: 10.1097/ALN.0000000000002774.
16. Ayoub J, Cohendy R, Prioux J, Ahmaidi S, Bourgeois JM, Dauzat M, et al. Diaphragm movement before and after cholecystectomy: a sonographic study. Anesth Analg 2001;92(3):755–761. DOI: 10.1097/00000539-200103000-00038.
17. Canet J, Gallart L, Gomar C, Paluzie G, Vallès J, Castillo J, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010;113(6):1338–1350. DOI: 10.1097/ALN.0b013e3181fc6e0a.
18. de Cleva R, de Assumpção MS, Sasaya F, Chaves NZ, Santo MA, Fló C, et al. Correlation between intra-abdominal pressure and pulmonary volumes after superior and inferior abdominal surgery. Clinics 2014;69(7):483–486. DOI: 10.6061/clinics/2014(07)07.
19. Treschan TA, Kaisers W, Schaefer MS, Bastin B, Schmalz U, Wania V, et al. Ventilation with low tidal volumes during upper abdominal surgery does not improve postoperative lung function. Br J Anaesth 2012;109(2):263–271. DOI: 10.1093/bja/aes140.
20. Bauer M, Opitz A, Filser J, Jansen H, Meffert RH, Germer CT, et al. Perioperative redistribution of regional ventilation and pulmonary function: a prospective observational study in two cohorts of patients at risk for postoperative pulmonary complications. BMC Anesthesiol 2019;19(1):132. DOI: 10.1186/s12871-019-0805-8.
21. Ferreyra GP, Baussano I, Squadrone V, Richiardi L, Marchiaro G, Del Sorbo L, et al. Continuous positive airway pressure for treatment of respiratory complications after abdominal surgery: a systematic review and meta-analysis. Ann Surg 2008;247(4):617–626. DOI: 10.1097/SLA.0b013e3181675829.
22. Nair J, Streeter KA, Turner SMF, Sunshine MD, Bolser DC, Fox EJ, et al. Anatomy and physiology of phrenic afferent neurons. J Neurophysiol 2017;118(6):2975–2990. DOI: 10.1152/jn.00484.2017.
23. Chae WS, Choi S, Sugiyama D, Richerson GB, Brennan TJ, Kang S. Effect of thoracic epidural anesthesia in a rat model of phrenic motor inhibition after upper abdominal surgery. Anesthesiology 2018;129(4):791–807. DOI: 10.1097/ALN.0000000000002331.
24. Clemente A, Carli F. The physiological effects of thoracic epidural anesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol 2008;74(10):549–563. PMID: 18854796.
25. Moraca RJ, Sheldon DG, Thirlby RC. The role of epidural anesthesia and analgesia in surgical practice. Ann Surg 2003;238(5):663–673. DOI: 10.1097/01.sla.0000094300.36689.ad.
26. Schober P, Vetter TR. Repeated measures designs and analysis of longitudinal data: if at first you do not succeed-try, try again. Anesth Analg 2018;127(2):569–575. DOI: 10.1213/ANE.0000000000003511.
27. Martinez BP, Silva JR, Silva VS, Neto MG, Forgiarini Júnior LA. Influence of different body positions in vital capacity in patients on postoperative upper abdominal. Brazilian J Anesthesiol 2015;65(3):217–221. DOI: 10.1016/j.bjane.2014.06.002.
28. Gea J, Gáldiz JB, Comtois N, Zhu E, Salazkin I, Fiz JA, et al. Changes in diaphragm activity induced by median laparotomy and changes in abdominal wall rigidity. Arch Bronconeumol 2009;45(1):30–35. DOI: 10.1016/j.arbres.2008.02.005.
________________________
© The Author(s). 2021 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.