ORIGINAL RESEARCH |
https://doi.org/10.5005/jp-journals-10071-24158 |
Accuracy of Estimated Continuous Cardiac Output Monitoring (esCCO) Using Pulse Wave Transit Time (PWTT) Compared to Arterial Pressure-based CO (APCO) Measurement during Major Surgeries
1,2Department of Anaesthesia, Critical Care and Pain, Tata Memorial Hospital, Mumbai, Maharashtra, India
3Division of Critical Care Medicine, Tata Memorial Centre, Homi Bhabha National Institute, Mumbai, Maharashtra, India
4Division of Critical Care Medicine, Department of Anaesthesia, Critical Care and Pain, Tata Memorial Centre, Homi Bhabha National Institute, Mumbai, Maharashtra, India
Corresponding Author: Atul P Kulkarni, Division of Critical Care Medicine, Department of Anaesthesia, Critical Care and Pain, Tata Memorial Centre, Homi Bhabha National Institute, Mumbai, Maharashtra, India, Phone: +91 9869077526, e-mail: kaivalyaak@yahoo.co.in
How to cite this article: Joshi M, Rathod R, Bhosale SJ, Kulkarni AP. Accuracy of Estimated Continuous Cardiac Output Monitoring (esCCO) Using Pulse Wave Transit Time (PWTT) Compared to Arterial Pressure-based CO (APCO) Measurement during Major Surgeries. Indian J Crit Care Med 2022;26(4):498–502.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Background: Pulse wave transit time is a novel method of estimating continuous cardiac output (esCCO). Since there are not many studies evaluating esCCO, we compared it with arterial pressure based cardiac output (APCO) method (FloTrac).
Methods: In this prospective single-center observational study, we included 50 adult patients planned to undergo supramajor oncosurgeries, where major blood loss and extensive fluid shifts were expected. Cardiac output (CO) measurements were obtained by both methods at five distinct time points, giving us 250 paired readings of stroke volume index (SVI) and cardiac index (CI). We analyzed these readings using Pearson’s correlation coefficient and Bland–Altman plots, along with other appropriate statistical tests.
Results: There was significant correlation between CI and SVI measured by the esCCO and APCO. Bland–Altman plot analysis for CI showed a bias of −0.44 L/minute/m2, precision of 0.74, and the limits of agreement of −1.89 and +1.01, while the percentage error was 46.29%. Bland–Altman analysis for SVI showed a bias −5.07 mL with a precision of 9.36, and the limits of agreement to be −23.4 to +13.28. The percentage error was 46.56%.
Conclusion: This study demonstrated that esCCO tended to underestimate the CI to a large degree, particularly while estimating the cardiac output in the lower range. We found that the limits of agreement between two methods were wide, which are not likely to be clinically acceptable. Further studies with larger number of data points, obtained in a similar subset of patients, for cardiac output measurement in the perioperative period will certainly help determine if pulse wave transit time (PWTT) is here to stay (CTRI No.: CTRI/2019/08/020543).
Keywords: Arterial pressure-based cardiac output, Bias, Estimated continuous cardiac output, Limits of agreement, Percentage error, Precision, Pulse wave transit time.
INTRODUCTION
Goal-directed fluid therapy (GDFT) advocates individualized fluid therapy using advanced monitoring for cardiovascular optimization and oxygen delivery by optimizing stroke volume index (SVI). Inappropriate administration of fluids may result either hypovolemia with decreased perfusion of major organs, or fluid overload, particularly in patients with compromised cardiovascular physiology.1,2 Studies show that fluid replacement guided by SVI optimization during major surgery reduces the volume of fluids infused, maintains intraoperative hemodynamic stability, improves perioperative gastrointestinal function, causes reduction in serum lactate at the end of surgery, and is associated with lower incidence of postoperative organ complications.3,4 Perioperative GDFT encompasses the following measures: identification of high-risk populations, starting hemodynamic therapy early in the preoperative period, optimization of fluids, vasopressors, and inotropes to achieve target blood flow and hemodynamic variables adjusted to the individual patient’s cardiovascular physiology and personal targets.5,6
No single cardiac output (CO) monitoring device has been shown to meet all clinical requirements. The complications associated with invasive monitoring have compelled the search for minimally invasive or noninvasive methods of CO measurement. Arterial pressure-based cardiac output (APCO), a noncalibrated CO measurement device, has been is in common clinical use since 2005, as FloTrac/Vigileo system (Edwards Lifesciences, Irvine, California). For measurement of APCO, arterial cannulation is essential for obtaining invasive arterial pressure (AP) waveform. The system using a proprietary algorithm measures the values of compliance and resistance of arteries and calculates continuous cardiac output. Use of APCO for GDFT has been shown to reduce postoperative complications and hospital length of stay (LOS) with improved outcomes.2 Pulse wave transit time (PWTT) is a novel, totally noninvasive technology, which estimates cardiac output (esCCO, Nihon Kohden, Tokyo, Japan) continuously. PWTT is the time from peak of R-wave to the rise point of the pulse oximeter wave and consists of three sub-time periods: pre-ejection period (PEP), pulse transit time through an elastic artery (from ascending aorta to pulse-oximetry probe site), and pulse transit time through peripheral resistance arteries (Fig. 1).7,8 PWTT displays the following parameters: estimated continuous cardiac output (esCCO), estimated stroke volume (esSV), and corresponding indices. PWTT has been compared with thermodilution, APCO measurement, and thoracic bioimpedance techniques in small number of patients undergoing various surgeries.9–11
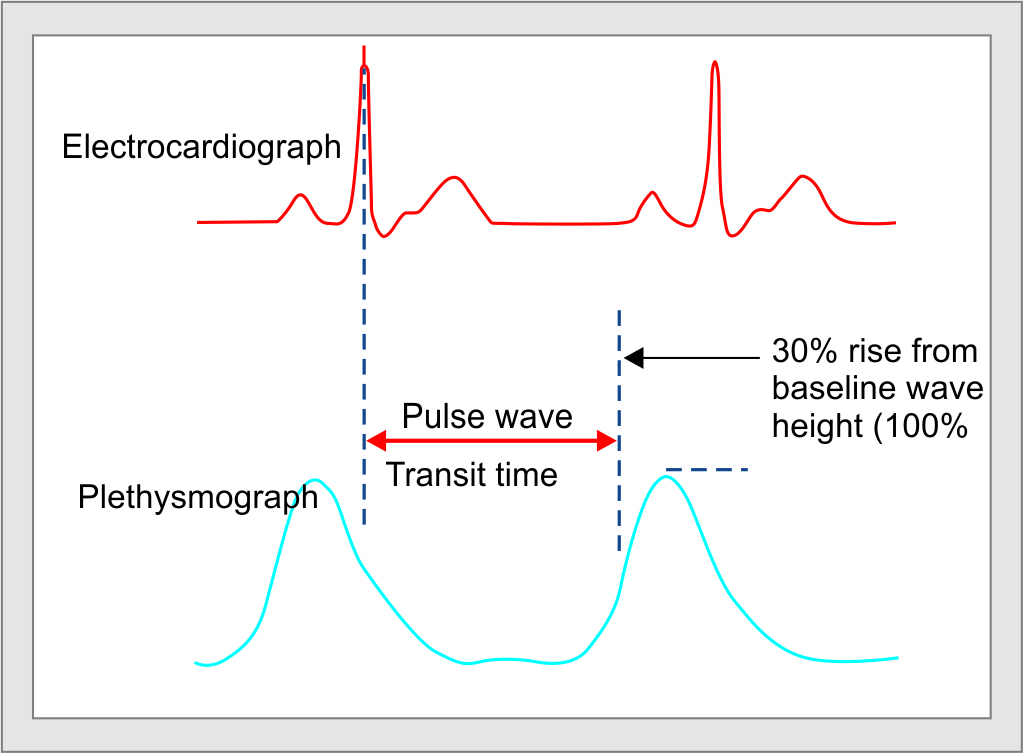
Fig. 1: Calculation of esCCO using PWTT from ECG and pulse oximetry waveforms
In this study, we therefore aimed to compare values of SVI and CI using arterial pressure-based CI with those obtained using PWTT technique in patients undergoing supramajor oncosurgeries. We hypothesized that if these values were similar, this will allow clinicians to use esCCO, a completely noninvasive monitor, in place of APCO, which needs arterial cannulation.
PATIENTS AND METHODS
This prospective observational study was conducted during October 2019–December 2020, after approval from the Institutional Ethics Committee (IEC) and registration with the Clinical Trials Registry of India. Written informed consent was obtained from the patients undergoing supramajor abdominal surgeries, which involved radical extensive resections with blood loss exceeding 500 mL and major fluids shifts, in a tertiary referral center.
All adult patients (>18 years) undergoing elective supramajor oncosurgeries under general anesthesia, who required placement of an arterial line, and in whom CO was monitored with FloTrac™ using EV1000™ monitor were included in the study. Younger patients (<18 years) and those with arrhythmias were excluded from the study.
After obtaining informed consent, we included 50 patients who fit the inclusion criteria. Demographic data of patients including age, sex, height, weight, BMI, and details of surgery were recorded. After inserting epidural catheter in the thoracic T8–9 interspace, anesthesia induction was left to the discretion of the OT anesthesiologist. Anesthesia was maintained with isoflurane 0.8–1.2% in 70:30 mixture of N2O and O2, All patients were paralyzed with intermittent boluses of vecuronium and ventilated using volume-control mode with tidal volume of 8 mL/kg of predicted bodyweight and 5 cm H2O of PEEP, and the peak pressure limit was set at 35 cm H2O, as per our institutional protocol. A FloTrac™ sensor with EV1000™ monitor was attached to the invasive arterial line. Leads for monitoring esCCO for VISMO® cardiac monitor were attached. The patient’s demographic data (height, weight, age, and gender) were entered into both the devices as per manufacturer’s recommendations. The hemodynamic parameters were recorded at various intervals as per the study design. We obtained 250 paired readings of SVI and cardiac index (CI) from FloTrac and esCCO monitors at different time points: baseline (T1), immediately after incision (T2), before (T3) and after fluid bolus (T4), and end of the surgery (T5). A maximum of five paired readings were obtained for each patient. Other variables recorded included heart rate (HR); systolic, diastolic, and mean AP (SBP, DBP, and MAP); SVI; stroke volume variation (SVV); and pulse pressure variation (PPV). We used stroke volume optimization strategy (fluid is infused, if the PPV >12%, till the stroke volume reaches a plateau) for all our patients, as per our institutional protocol, with an aim to maintain a urine output of 0.5–1 mL/kg/hour. The patients were given a fluid bolus (350 mL of Ringer lactate over 10 minutes), if there were one or more signs of acute circulatory failure, such as tachycardia with SBP <90 mm Hg or need for vasopressors, or oliguria (urine output <0.5 mL/kg/minute for at least two hours), or lactate levels >2 mmol/L.
STATISTICS
Sample size calculation: We needed 250 paired readings to achieve >80% power (83%) to detect agreement between two methods when the confidence level of the limits of agreement was 0.950. The maximum allowable difference was taken to be 2.850. The mean and standard deviation of the sample differences were anticipated to be −0.700 and 0.800. IEC wanted us to record not more than five readings per patient; we therefore included 50 patients in the study.
Student’s t-test, intraclass correlation, and Pearson’s correlation coefficient were used for normally distributed, continuous/quantitative variables (expressed as mean ± SD), whereas Chi-square test was performed for categorical/qualitative variables (expressed as numbers and percentage). Mann–Whitney test was utilized for non-Gaussian data (expressed as median and range). p <0.05 was considered statistically significant. We performed correlation analyses using Pearson’s correlation coefficient for both SVI and CI using PWTT and APCO methods. We then constructed Bland–Altman plots to determine the bias (mean differences between esCCO and APCO) and precision (one standard deviation of this difference). The upper and lower limits of agreement were calculated as the bias ±2 SD. 95% limits of agreement were denoted by the interval defined by the observed bias ±1.96 the observed SD of the observed differences. The percentage error was calculated using the ratio of 2 SD of the bias to the mean and was considered clinically acceptable if that result was ≤30%, as proposed by Critchley and Critchley.12 Statistical analyses were performed using IBM SPSS (International Business Machines; Statistical Package for the Social Sciences) statistics for windows (version 23.0; released 2015; Armonk, New York: IBM Corp.).
RESULTS
In this prospective observational study, we obtained 250 paired measurements of CI and SVI at five different time points using the PWTT and arterial pressure-based CO measurement techniques (FloTrac™). The correlation analysis demonstrated a significant correlation between esCCI and CI by APCO (r = 0.632, p = 0.001), and coefficient of determination was r2 = 0.399 (Fig. 2). Similarly, SVI measured by two methods showed significant correlation (r = 0.469, p = 0.001) and coefficient of determination was r2 = 0.220 (Fig. 3).
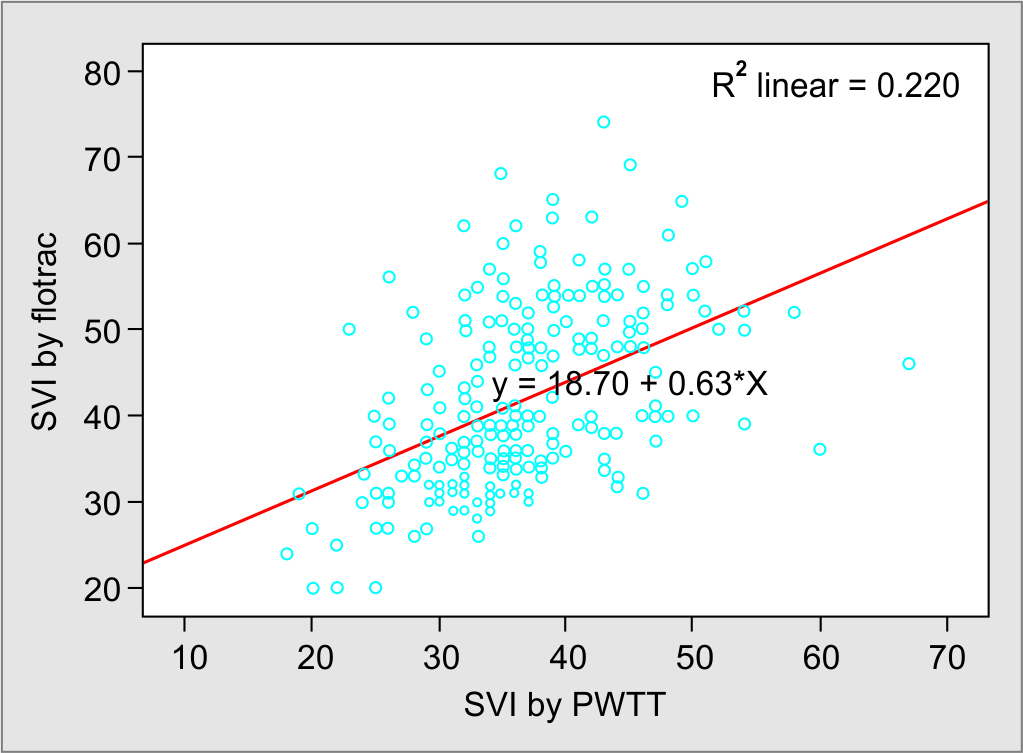
Fig. 2: Scatter plot for SVI using PWTT and APCO measurement
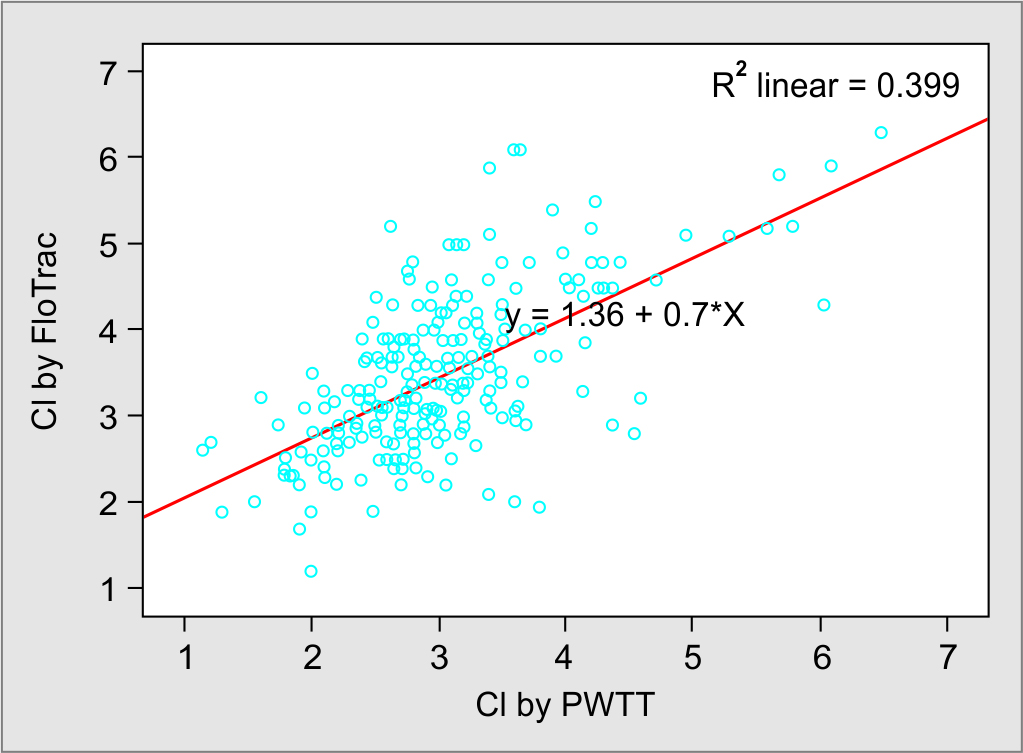
Fig. 3: Scatter plot for CI using PWTT and APCO measurement
On Bland–Altman plot, for the CI, the bias was −0.44 L/minute/m2, precision was 0.74, and the limits of agreement measured by the two methods were −1.89 to +1.01. The percentage error was 46.29% (Fig. 4). Bland–Altman plot for SVI measured by two methods showed bias of 5.07 mL and precision of 9.36, the limits of agreement were −23.4 to +13.28, and percentage error was 46.56% (Fig. 5).
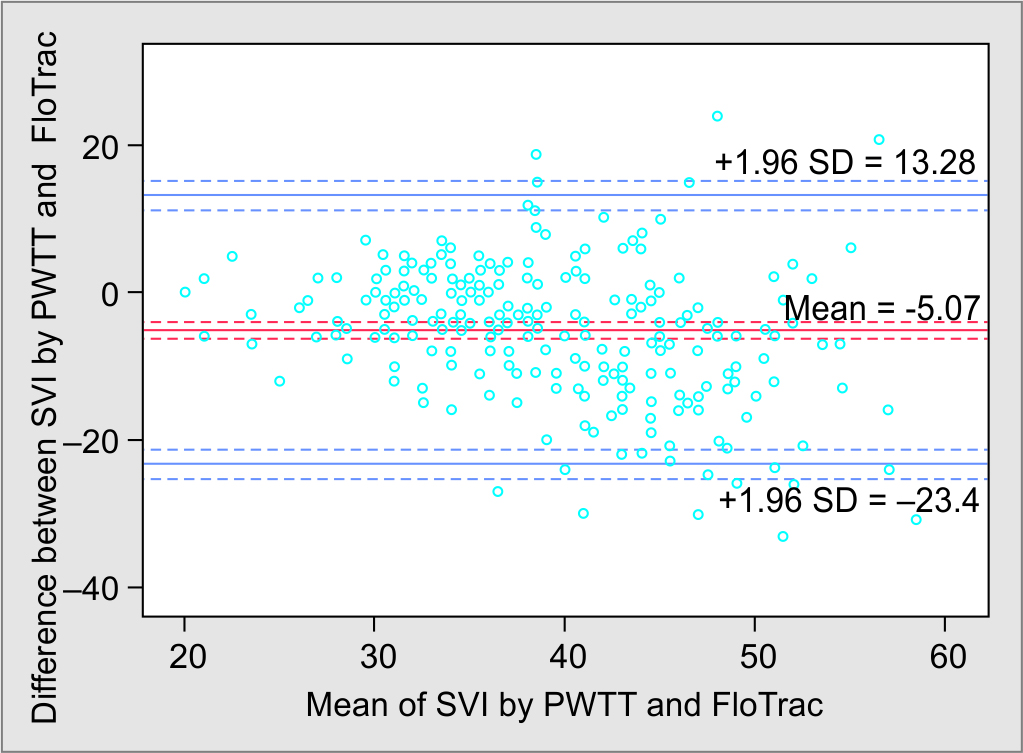
Fig. 4: Limits of agreement between SVI using PWTT and APCO measurement

Fig. 5: Limits of agreement between CI using PWTT and APCO measurement
DISCUSSION
In our prospective observational study of 50 patients undergoing major surgeries, there was a significant correlation between estimated CI as well as SVI, measured by the two methods. As per the Bland–Altman plot for CI, PWTT tended to underestimate the CI by −0.44 L/minute/m2, and the limits of agreement were wide. The percentage error for CI was 46.29% in our study which is very high, against the recommendation of Cecconi et al., who mentioned that finding a percentage error up to >30% for a cardiac output monitor was clinically unacceptable.13 This means that the new technique has an error which exceeds that of the reference technique, which therefore cannot replace the old method. In our cohort, the percentage error for both CI and SVI was above 45%. It must be mentioned here that Cecconi et al. used cardiac output obtained by intermittent thermodilution using pulmonary artery catheter (PAC).13 PAC is still considered the gold standard method for measurement of CO; however, its use in clinical practice has substantially decreased due to its invasive nature, likelihood of complications, and need for special skill for insertion as well as interpretation of data. In our institute, we now rarely use PAC, while FloTrac™ is routinely used for measurement of CO/CI. However, FloTrac™ needs arterial cannulation, while pulse wave transit time (PWTT) is completely noninvasive, with no possible harm to the patients. Thus, PWTT would be a pragmatic substitute for FloTrac, if proved to be accurate. We therefore compared CI measured by PWTT with FloTrac™.
There have been several other studies, which have attempted to find the correlation between estimated cardiac output using PWTT and other cardiac output measurement techniques, with conflicting results. Yamada et al. compared the accuracy of estCCO and thermodilution cardiac output (TDCO), in 213 ICU and OT patients, in whom 587 esCCO and TDCO datasets were obtained.7 The correlation coefficient was 0.79 (p <0.0001, 95% confidence limits of 0.756–0.819), with a small bias (mean difference between esCCO and TDCO) of 0.13 L/minute (95% confidence interval of bias 0.04–0.22 L/minute), and the precision (1 SD) was 1.15 L/minute (95% prediction interval was −2.13 to 2.39 L/minute). They concluded that the CO measured by both methods showed close correlation with small bias and precision, and these findings differ from our study. Thus, the esCCO method was comparable to arterial waveform analysis technologies in current use. In another study, Terada et al. compared the esCCO system (PWTT) for noninvasive measurement and an APCO system for evaluating changes in CO among patients posted for laparotomy without postural change.8 The correlation analysis between two techniques showed the coefficient to be 0.72. Using a Bland–Altman plot to compare APCO and esCCO, they noted a bias of 0.75, the precision was 0.86 L/minute, and the percentage error was 41%. They inferred that the interchangeability of esCCO with APCO is not possible. However, the trends of both measurements were similar, as determined by polar plot analysis. Magliocca et al., compared CO measured by PWTT and thoracic bioimpedance (ICON; Osypka Medical GmbH, Berlin, Germany) technologies with thermodilution CO measured with pulmonary artery catheter, in patients undergoing orthotopic liver transplant.10 They found that the PWTT and thoracic bioimpedance had limited accuracy as noninvasive CO estimation with esCCO and ICON exhibited limited accuracy and precision, mean bias for esCCO 2.0 L/minute (SD, ±2.7 L/minute) and −3.3 L/minute (SD, ±2.8 L/minute) for ICON compared to TDCO. They suggested that both noninvasive techniques showed trend similar to TDCO. However, both were largely inaccurate, despite with reasonable trending ability. The errors increased particularly when there were large changes in systemic vascular resistance and arterial elastance. A study from Thailand compared esCCO and APCO to TDCO in 50 adult Thai patients undergoing cardiac surgery with cardiopulmonary bypass.14 In comparison with TDCO, both techniques overestimated CO, though they trended in the same direction as TDCO.
The novelty of PWTT monitoring is its noninvasiveness and the continuous assessment of cardiac output and stroke volume. Continuous detection of these values may help in timely detection of fluid requirement and may avoid invasive monitoring.
The strength of our study is that we compared esCCO and APCO technologies for CO measurements in patients who were undergoing supramajor oncosurgeries, where significant blood loss and major fluid shifts are common. We also compared these methods at various intervals which are significant during the intraoperative periods such as when patients were likely to be hypovolemic or had just received fluid boluses for treatment of hypotension, both instances which can cause significant changes in venous and arterial tone, compliance, and elastance. These instabilities in the hemodynamic condition of the patients would probably explain the wide limits of agreement we found in our study. The limitation of our study is that this is a single-center study, and we did not compare esCCO to the TDCO obtained by the pulmonary artery catheter. However, we feel that this is of small consequence as the pulmonary artery catheter is nowadays not commonly used.
CONCLUSION
This study demonstrated that esCCO tended to underestimate the CI to a large degree, particularly while estimating the cardiac output in the lower range. We found that the limits of agreement between two methods were wide, which are not likely to be clinically acceptable. Further studies with larger number of data points, obtained in a similar subset of patients, for cardiac output measurement in the perioperative period will certainly help determine if PWTT is here to stay.
ORCID
Malini Joshi https://orcid.org/0000-0001-5051-9474
Resham Rathod https://orcid.org/0000-0003-1403-3658
Shilpushp J Bhosale https://orcid.org/0000-0002-0290-0526
Atul P Kulkarni https://orcid.org/0000-0002-5172-7619
REFERENCES
1. Myles P, Bellomo R, Corcoran T, Forbes A, Wallace S, Peyton P, et al. Australian and New Zealand College of Anaesthetists Clinical Trials Network, and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Restrictive versus liberal fluid therapy in major abdominal surgery (RELIEF): rationale and design for a multicentre randomised trial. BMJ Open 2017;7(3):e015358. DOI: 10.1136/bmjopen-2016-015358.
2. Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care 2010;14(3):R118. DOI: 10.1186/cc9070.
3. Cecconi M, Monti G, Hamilton MA, Puntis M, Dawson D, Tuccillo ML, et al. Efficacy of functional hemodynamic parameters in predicting fluid responsiveness with pulse power analysis in surgical patients. Minerva Anestesiol 2012;78(5):527–533. PMID: 22534733.
4. Mayer J, Boldt J, Mengistu AM, Röhm KD, Suttner S. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Crit Care 2010;14(1):R18. DOI: 10.1186/cc8875.
5. Saugel B, Kouz K, Scheeren TWL. The ‘5 Ts’ of perioperative goal-directed haemodynamic therapy. Br J Anaesth 2019;123(2):103–107. DOI: 10.1016/j.bja.2019.04.048.
6. Salzwedel C, Puig J, Carstens A, Bein B, Molnar Z, Kiss K, et al. Perioperative goal-directed hemodynamic therapy based on radial arterial pulse pressure variation and continuous cardiac index trending reduces postoperative complications after major abdominal surgery: a multi-center, prospective, randomized study. Crit Care 2013;17(5):R191. DOI: 10.1186/cc12885.
7. Yamada T, Tsutsui M, Sugo Y, Sato T, Akazawa T, Sato N, et al. Multicenter study verifying a method of noninvasive continuous cardiac output measurement using pulse wave transit time: a comparison with intermittent bolus thermodilution cardiac output. Anesth Analg 2012;115(1):82–87. DOI: 10.1213/ANE.0b013e31824e2b6c.
8. Terada T, Oiwa A, Maemura Y, Robert S, Kessoku S, Ochiai R. Comparison of the ability of two continuous cardiac output monitors to measure trends in cardiac output: estimated continuous cardiac output measured by modified pulse wave transit time and an arterial pulse contour-based cardiac output device. J Clin Monit Comput 2016;30(5):621–627. DOI: 10.1007/s10877-015-9772-x.
9. Tsutsui M, Araki Y, Masui K, Kazama T, Sugo Y, Archer TL, et al. Pulse wave transit time measurements of cardiac output in patients undergoing partial hepatectomy: a comparison of the esCCO system with thermodilution. Anesth Analg 2013;117(6):1307–1312. DOI: 10.1213/ANE.0b013e3182a44c87.
10. Magliocca A, Rezoagli E, Anderson TA, Burns SM, Ichinose F, Chitilian HV. Cardiac output measurements based on the pulse wave transit time and thoracic impedance exhibit limited agreement with thermodilution method during orthotopic liver transplantation. Anesth Analg 2018;126(1):85–92. DOI: 10.1213/ANE.0000000000002171.
11. Terada T, Kessoku S, Suzuki A, Kurosawa A, Nakagomi S, Oiwa A, et al. Comparison of the pulse wave transit time method and an arterial pressure-based cardiac output system for measuring cardiac output trends during laparotomy without postural change. Asian J Anesthesiol 2019;57(3):85–92. DOI: 10.6859/aja.201909_57(3).0003.
12. Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 1999;15(2):85–91. DOI: 10.1023/a:1009982611386.
13. Cecconi M, Rhodes A, Poloniecki J, Della Rocca G, Grounds RM. Bench-to-bedside review: the importance of the precision of the reference technique in method comparison studies–with specific reference to the measurement of cardiac output. Crit Care 2009;13(1):201. DOI: 10.1186/cc7129.
14. Wacharasint P, Kunakorn P, Pankongsap P, Preechanukul R. Clinical validation of pulse contour and pulse wave transit time-based continuous cardiac output analyses in Thai patients undergoing cardiac surgery. J Med Assoc Thai 2014;97 Suppl 1:S55–S60. PMID: 24855843.
________________________
© The Author(s). 2022 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.